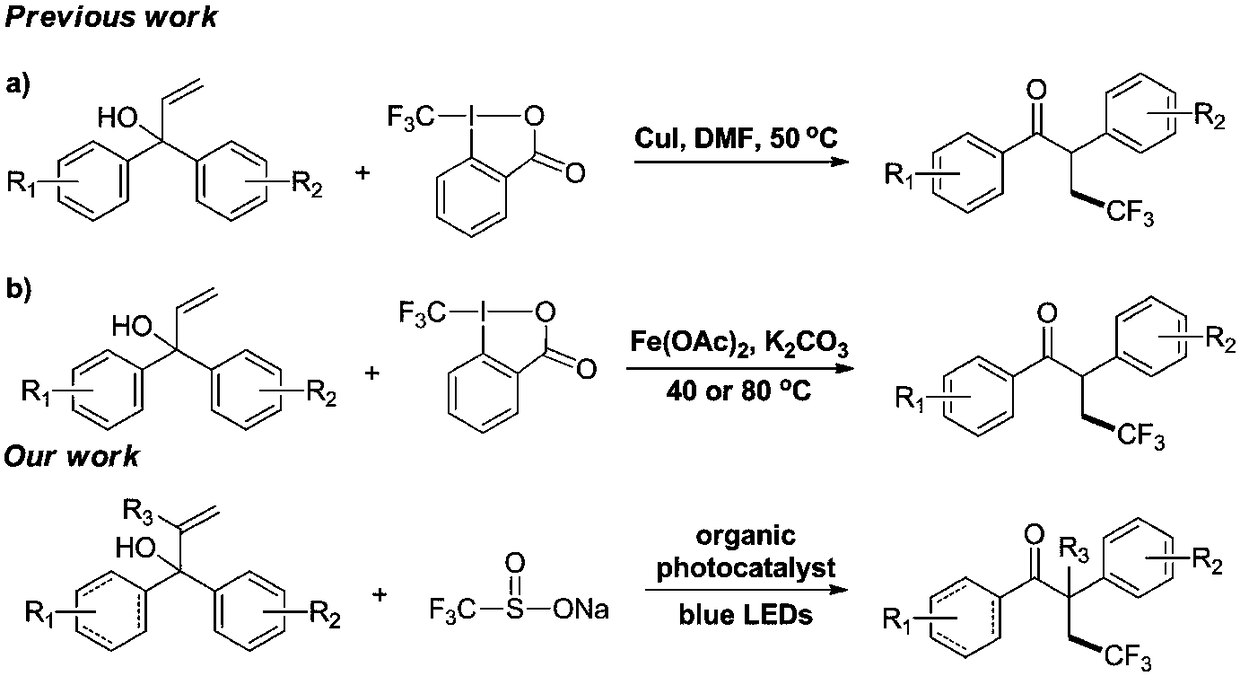
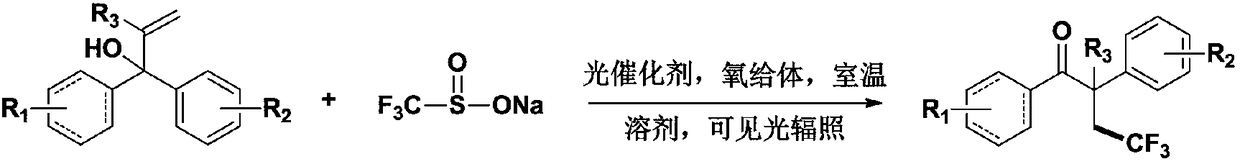
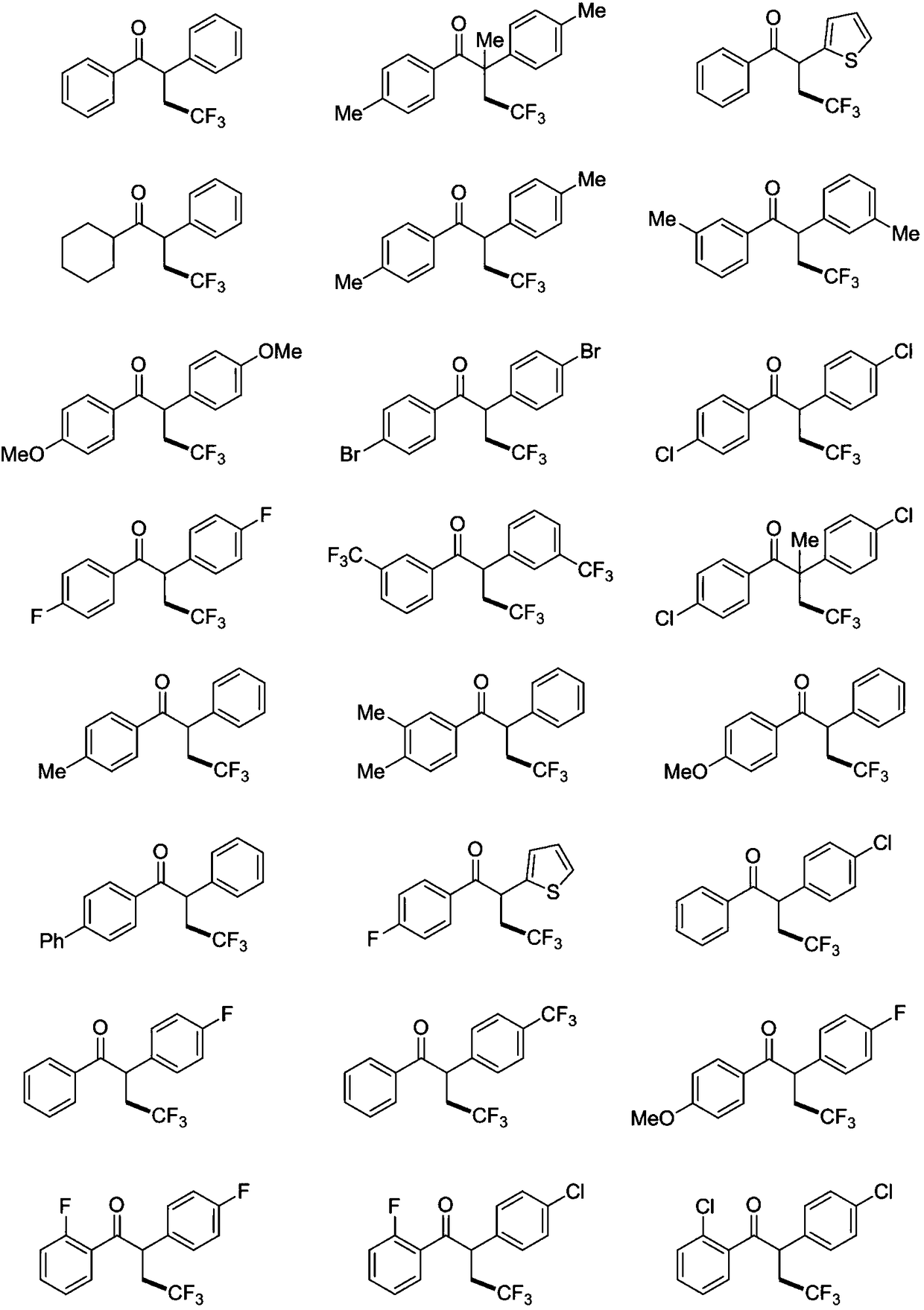
Method for preparing alpha-aryl-beta-trifluoromethyl ketone compound through visible light catalysis
A trifluoromethyl ketone, a technology for catalytic preparation is applied in the field of visible light catalytic preparation of α-aryl-β-trifluoromethyl ketone compounds, and can solve the problems of unstable chemical properties, high reaction temperature, high price and the like, Achieve the effect of low price, stable chemical properties and strong substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for preparing 4,4,4-trifluoro-1,2-diphenyl-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula:
[0025]
[0026] Preparation of 4,4,4-trifluoro-1,2-diphenyl-1-butanone: Add 2.1 g of 1,1-diphenyl to a 200 ml dry single-necked bottle in the presence of atmospheric pressure of air Phenyl-2-propen-1 alcohol (10 mmol), 3.12 g of sodium trifluoromethylsulfinate (20 mmol), 0.158 g of 1,2,3,4-tetrakis(carbazol-9-yl )-4,6-dicyanobenzene (0.2 mmol) and 80 ml of 1,2-dichloroethane. Place the reaction solution at room temperature under 18-watt blue LED light irradiation (the distance between the reaction bottle and the light source is about 10-15 cm) until 1,1-diphenyl-2-propen-1 alcohol completely disappears (TLC track). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rotary evaporation, and the obtained crude product was separated by column chromatography (eluent: n-hexane / et...
Embodiment 2
[0030] A method for preparing 4,4,4-trifluoro-2-methyl-1,2-bis(4-methyl-phenyl)-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula :
[0031]
[0032]The preparation of 4,4,4-trifluoro-2-methyl-1,2-di(4-methyl-phenyl)-1-butanone: In the presence of atmospheric pressure air, pour into a 200 ml dry Add 2.52 grams of 2-methyl-1,1-bis(4-methyl-phenyl)-2-propen-1-ol (10 mmol), 3.12 grams of trifluoromethylsulfinic acid successively in the single-necked bottle Sodium (20 mmol), 0.158 g of 1,2,3,4-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (0.2 mmol) and 80 ml of 1,2-dichloro ethane. At room temperature, place the reaction solution under an 18-watt blue LED light (the distance between the reaction bottle and the light source is about 10-15 cm) until 2-methyl-1,1-bis(4-methyl-phenyl )-2-propen-1-ol disappeared completely (TLC trace). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rotary...
Embodiment 3
[0036] A method for preparing 4,4,4-trifluoro-1-phenyl-2-(2-thienyl)-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula:
[0037]
[0038] Preparation of 4,4,4-trifluoro-1-phenyl-2-(2-thienyl)-1-butanone: In the presence of atmospheric pressure air, add 2.16 g 1-phenyl-1-(2-thienyl)-2-propen-1-ol (10 mmol), 3.12 g of sodium trifluoromethylsulfinate (20 mmol), 0.158 g of 1,2 , 3,4-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (0.2 mmol) and 80 ml of 1,2-dichloroethane. At room temperature, place the reaction solution under an 18-watt blue LED light (the distance between the reaction bottle and the light source is about 10-15 cm) until 1-phenyl-1-(2-thienyl)-2-propene- The 1-ol disappeared completely (TLC trace). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rotary evaporation, and the obtained crude product was separated by column chromatography (eluent: n-hexane / ethyl acetate=10:1-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com