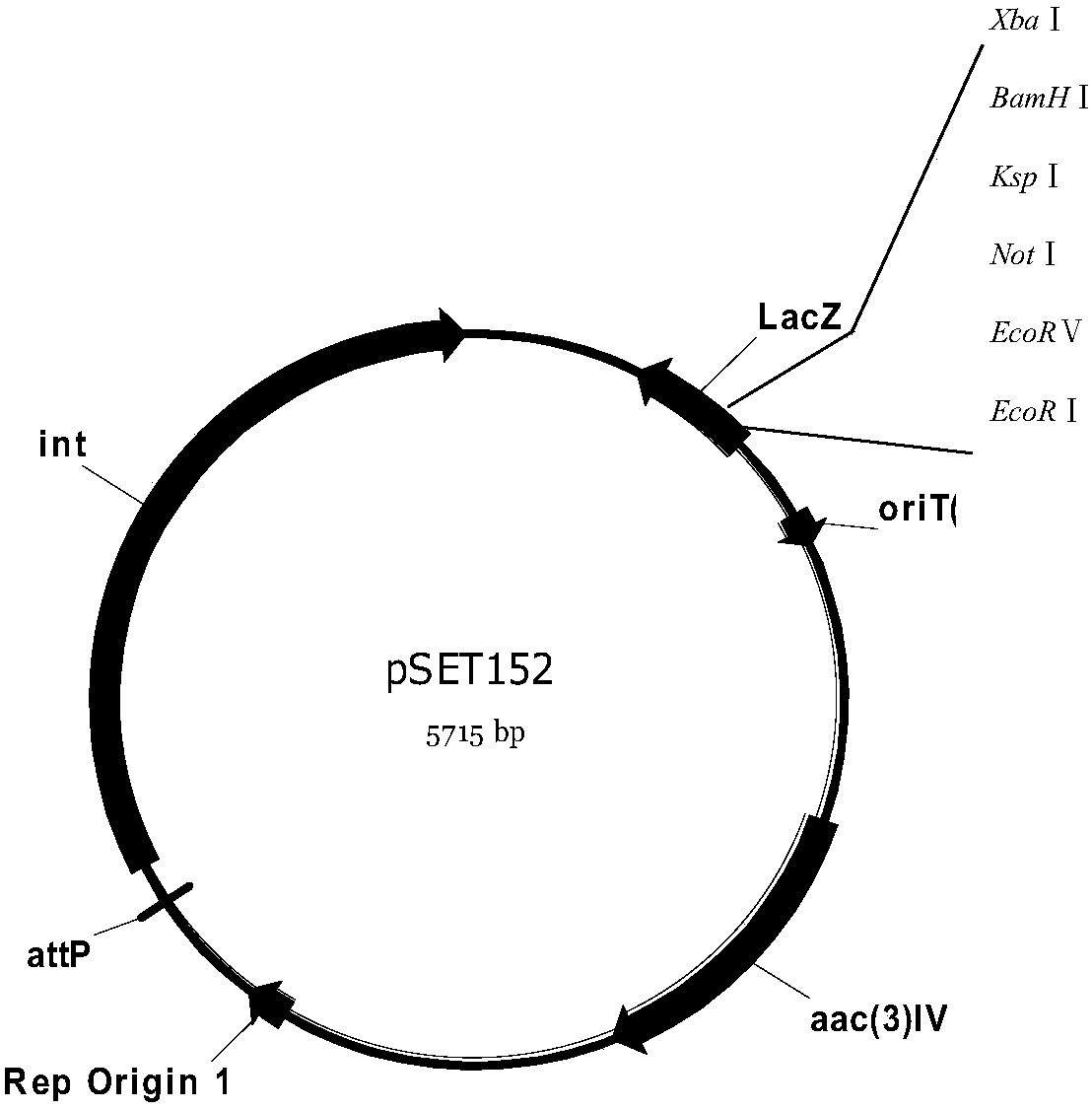
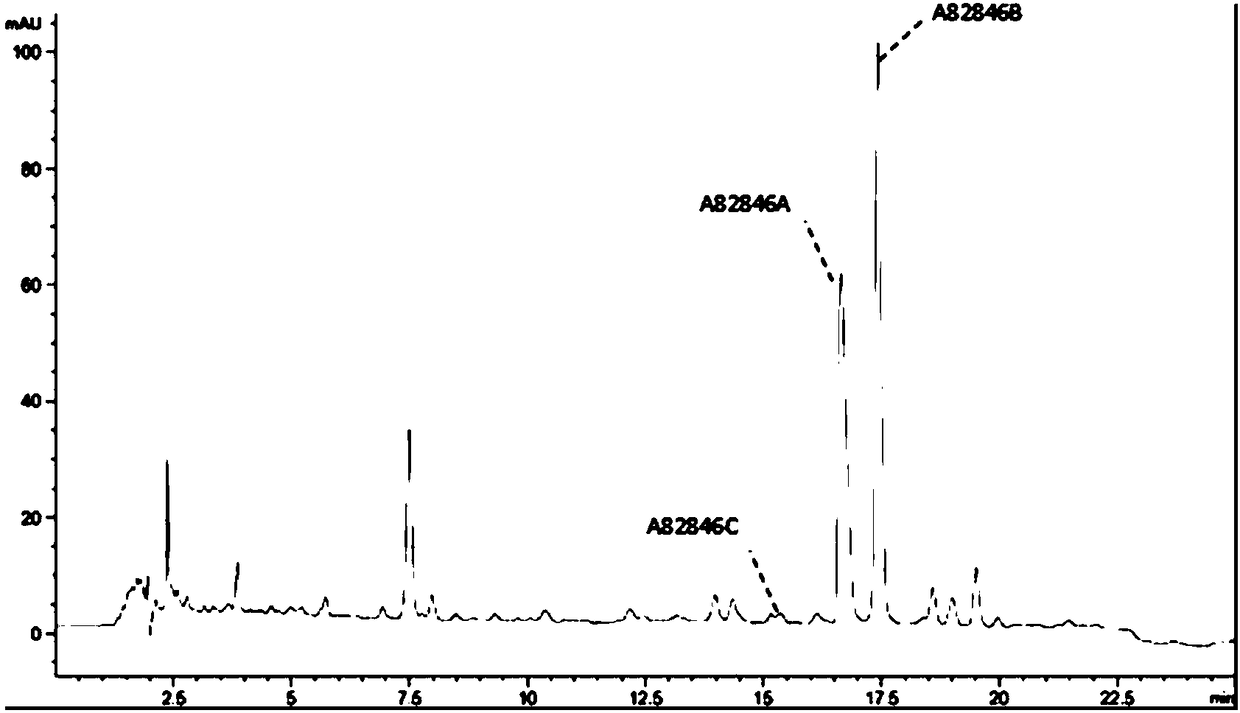
Genetically engineered bacterium for producing A82846B as well as preparation method and application of genetically engineered bacterium
A genetically engineered bacteria and gene technology, which is applied in the field of genetically engineered bacteria for the production of A82846B and its construction/preparation, can solve the problems of increased time and economic costs for separation and purification, and achieve the advantages of reducing production costs, improving production efficiency, and reducing environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Amplification, Cloning and Expression of Endogenous Halogenase Gene chaA
[0036](1) Genome extraction of Amycolatopsis orientalis strain NRRL 18099 (purchased from NRRL) and amplification of endogenous halogenase gene chaA:
[0037] NRRL 18099 was inoculated in 25ml of TSB (Shanghai Yuanju Biological Co., Ltd.) medium, and cultured at 28°C and 220rpm shaking (Shanghai Zhichu Instrument Co., Ltd.) for 48h. The culture solution was centrifuged (Hunan Xiangyi Instrument Development Co., Ltd.) at 12,000 rpm for 5 minutes, the supernatant was poured out, and the bacteria were recovered. Using the Bacterial Genomic DNA Rapid Extraction Kit, the genome was extracted according to the extraction method provided by the supplier (Shanghai Jierui Bioengineering Co., Ltd.).
[0038] The primers were synthesized by Shanghai Bailige Biotechnology Co., Ltd., and the design is as follows:
[0039] ChaA-F: 5'-CATATGATGTCGGTCGAAGACTTCGATGTGG-3' (see SEQ ID NO.6);
[0040] ChaA-R: 5'-G...
Embodiment 2
[0062] Amplification, cloning and expression of exogenous halogenase gene
[0063] (1) Total gene synthesis of exogenous halogenase gene
[0064] Exogenous halogenase genes include the genes of halogenases with high homology to their own halogenase obtained by Blast amino acid sequence alignment: vancomycin halogenase synthesis gene vcm8, balhimycin halogenase gene bhaA, teicoplanin halogenase synthesis gene thaA, etc. Listed in Table 3 below.
[0065] Table 3. The source and number of strains of exogenous halogenase gene
[0066]
[0067] Splice the gene vcm8 sequence in the above table with the terminator sequence (SEQ ID No.10), add an NdeI restriction site at the beginning of the target gene sequence, and add a BamHI restriction site at the end of the terminator sequence to form NdeI The sequence of -vcm8-terminator-BamHI sequence was sent to Beijing Weishang Lide Biotechnology Co., Ltd. for whole gene synthesis, and connected to PWSV plasmid to obtain PWSV-vcm8. In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com