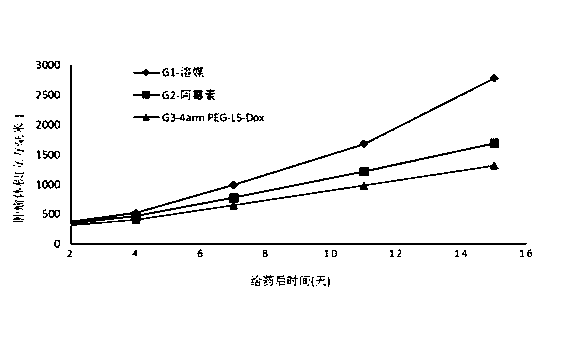
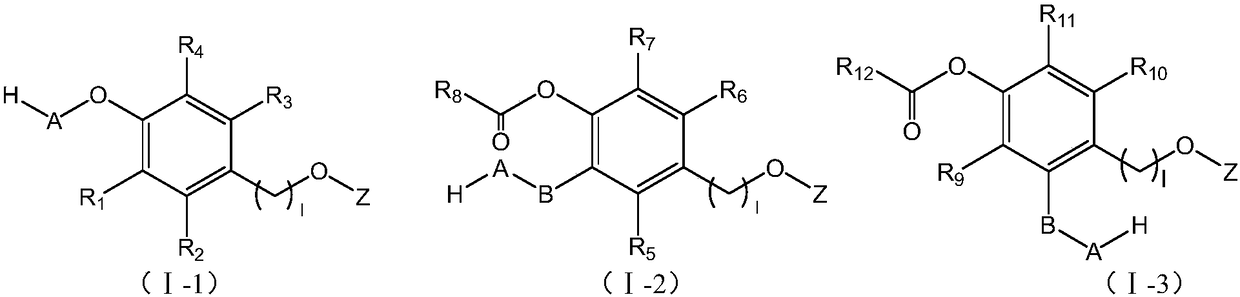
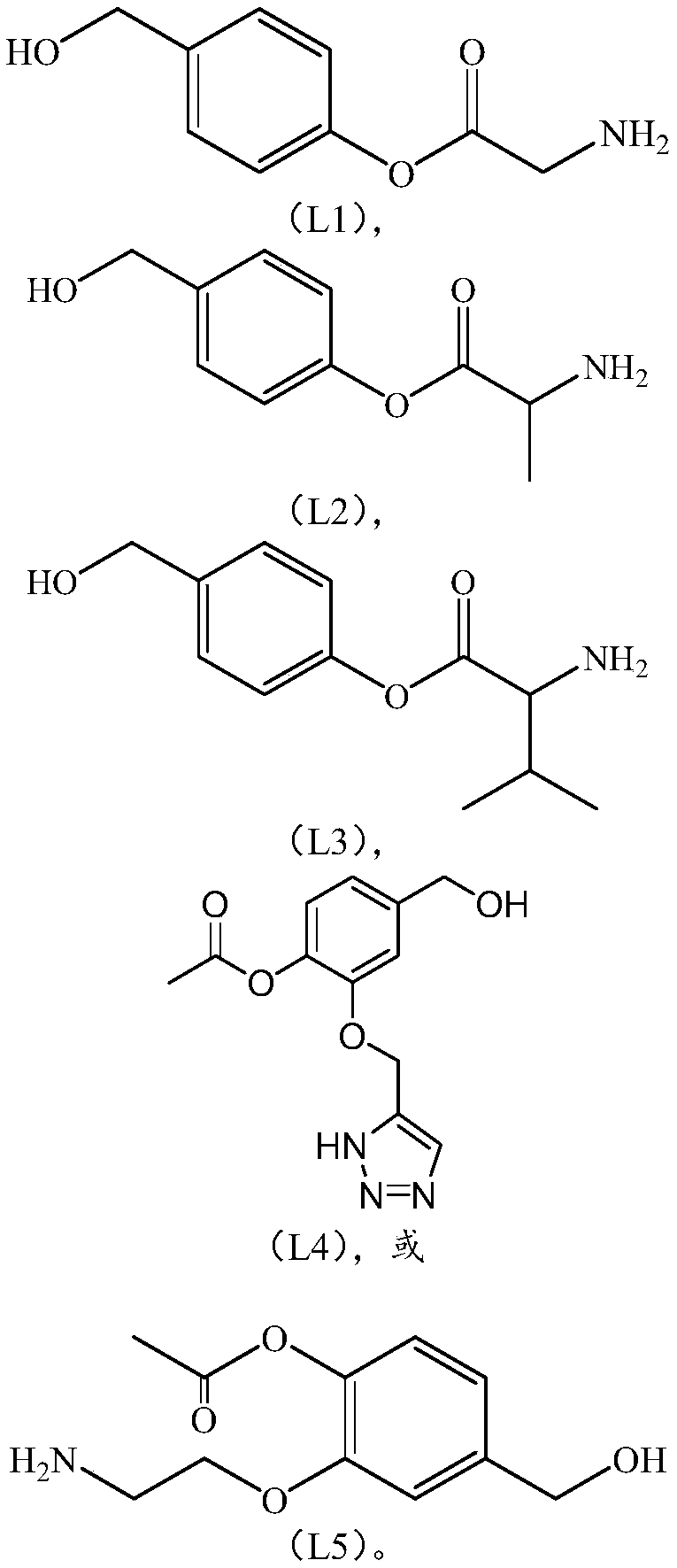
Linker compound, polyethylene glycol-linker compound and derivatives thereof and polyethylene glycol-linker-drug conjugate
A polyethylene glycol and compound technology, applied in the field of medicine, can solve the problems of low bioavailability, unfavorable patient compliance, reducing drug immunogenicity and toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1 Synthesis of Linking Chain (L)
[0182]
[0183] BOC-amino acid (92.2 mmol) and N,N-dicyclohexylcarbodiimide (DCC, 23.8 g, 115.3 mmol) were added to dichloromethane (500 mL), cooled in an ice-water bath, and p-hydroxybenzyl alcohol ( 11.4 g, 92.2 mmol), remove the ice bath after the addition, and react at room temperature overnight. Filtration, the filter cake was washed with ethyl acetate, and the filtrate was evaporated to dryness to obtain the crude product, which was purified by column chromatography to obtain product 1.
[0184] 1a: 19.7 g, yield 76.0%. 1 H NMR: (CDCl 3 ): 8.75(s, 1H), 7.22(d, 2H), 7.05(d, 2H), 4.87(s, 2H), 3.74(s, 2H), 1.52(s, 9H).
[0185] 1b: 20.3 g, yield 74.8%. 1 H NMR: (CDCl 3 ): 8.74(s, 1H), 7.21(d, 2H), 7.05(d, 2H), 4.88(s, 2H), 3.77(m, 1H), 1.51(s, 9H), 1.27(d, 3H) .
[0186] 1c: 21.6 g, yield 72.5%. 1 H NMR: (CDCl 3 ): 8.75(s, 1H), 7.22(d, 2H), 7.05(d, 2H), 4.87(s, 2H), 3.61(d, 1H), 2,82(m, 1H), 1.52(s, 9H), 1.06(d,...
Embodiment 2
[0191] Example 2 Synthesis of the combination of monomethoxy polyethylene glycol acetic acid and linking chain (mPEG-L(40K))
[0192]
[0193] Monomethoxy polyethylene glycol-acetic acid (mPEG-CM, 40K, 5 g, 0.125 mmol), compound L (0.25 mmol, prepared in Example 1)
[0194] and 1-hydroxybenzotriazole (HOBt, 16.9 mg, 0.125 mmol) were added to the reaction flask, dissolved in dichloromethane, and then diisopropylethylamine (45.2 mg, 0.35 mmol) was added, stirring uniformly, After cooling in an ice bath, (EDCI, 47.9 mg, 0.25 mmol) was added in batches. After the addition, the system naturally rose to room temperature, and the reaction was carried out overnight. The next day after concentration, the residue was crystallized with isopropanol, filtered with suction, and dried to obtain the product mPEG-L.
[0195] mPEG-L1 (40K): 4.6 g, yield 92.4%.
[0196] mPEG-L2 (40K): 4.5 g, yield 90.8%.
[0197] mPEG-L3 (40K): 4.7 g, yield 93.7%.
Embodiment 3
[0198] Example 3 Preparation of monomethoxy polyethylene glycol-doxorubicin conjugate (mPEG-L-Dox(40K))
[0199]
[0200] Compound mPEG-L (0.075 mmol, prepared in Example 2) was added to the reaction flask, dissolved in dichloromethane (30 mL), N 2 Cool under protection, add succinimidyl carbonate (23.0 mg, 0.09 mmol), stir to dissolve, then add triethylamine (10.1 mg, 0.1 mmol), remove the cooling bath after the addition, and react at room temperature overnight. The reaction solution was concentrated, and the residue was crystallized with isopropanol to obtain the product mPEG-L-NHS.
[0201] mPEG-L1-NHS (40K): 2.6 g, yield 88.5%.
[0202] mPEG-L2-NHS (40K): 2.7 g, yield 89.2%.
[0203] mPEG-L3-NHS (40K): 2.6 g, yield 87.9%.
[0204] Compound mPEG-L-NHS (0.06 mmol, prepared above) was dissolved in dichloromethane (25 mL), N 2 Cool under protection, add diisopropylethylamine (12.9 mg, 0.1 mmol), stir evenly, then add doxorubicin hydrochloride (52.2 mg, 0.09 mmol), and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com