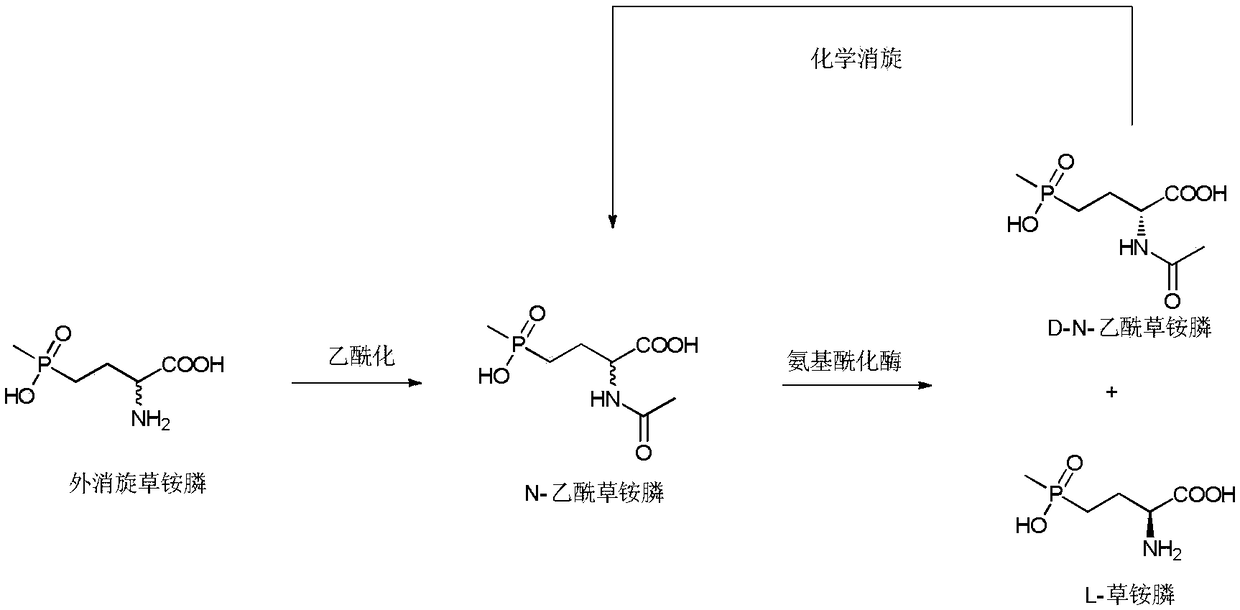
Method of utilizing chemistry-enzyme method to produce L-glufosinate ammonium
A technology of glufosinate-ammonium and enzymatic method, which is applied in the field of chemical-enzymatic production of L-glufosinate-ammonium, which can solve the problems of reduced yield and achieve the effects of easy separation, simple reaction steps and high optical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of racemic N-acetylglufosinate-ammonium
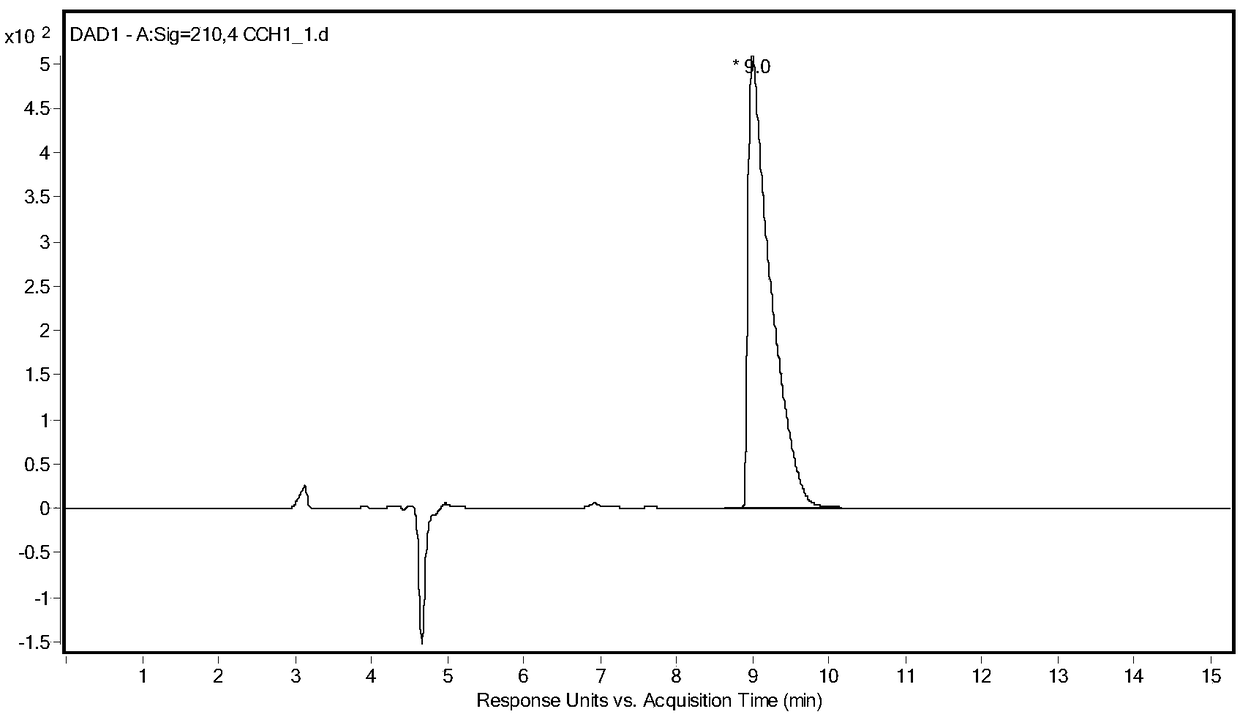
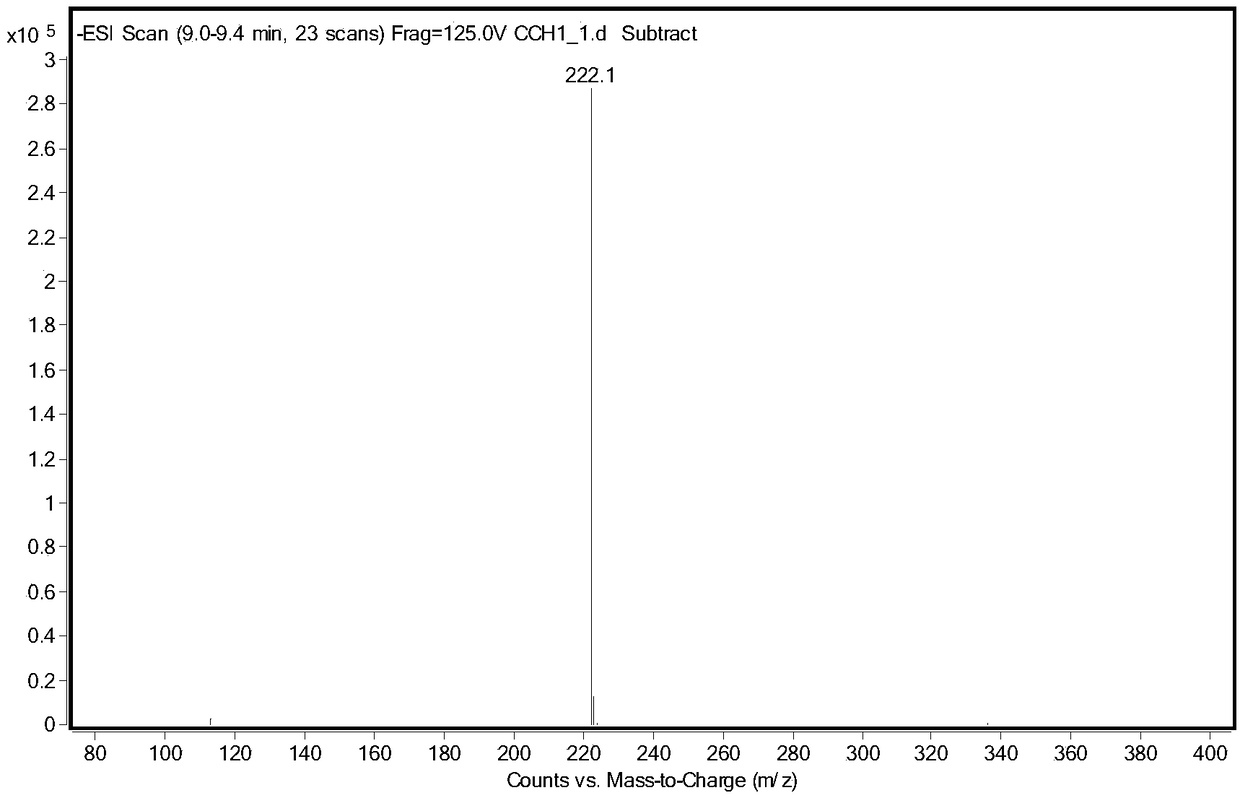
[0026] Take 5g of racemic glufosinate-ammonium, put it into a 250mL three-neck flask, add 100g of acetic acid, keep stirring at 40°C, add 5g of acetic anhydride, and react until the solution is clear and transparent. Rotary evaporator 80 ℃, vacuum 0.075 ~ 0.085MPa, evaporate acetic acid. The concentrate was dissolved in water, and the aqueous sodium hydroxide solution was adjusted to neutrality (pH 8.5), and ethanol was added to stand still to precipitate crystals, and after drying, racemic N-acetylglufosinate-ammonium was obtained. Utilize liquid chromatography (Shimadzu LC-16) to detect, the liquid phase spectrogram is figure 2 , mass spectrometry confirmed that it was N-acetylglufosinate-ammonium ( image 3 ). The column is C-18column (250mm×4.6mm, 5μm), the mobile phase is 10mM phosphoric acid solution (pH 2.1), the flow rate is 0.75mL / min, the ultraviolet detection wavelength is 210nm, and ...
Embodiment 2
[0027] Embodiment 2: the construction of engineering bacteria
[0028] A strain derived from Stenotrophomonas sp., annotated as the sequence of carboxypeptidase Ss1, was codon-optimized for whole gene synthesis, and the expression plasmid was pET-28b. The stop codon was site-directedly mutated so that the tail (C-terminus) of the expressed recombinase contained the histidine tag in pET28b. The inserted sequence (the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2) was verified by sequencing and then transferred into the expression host E. coli BL21 (DE3) for subsequent recombinase expression.
Embodiment 3
[0029] Embodiment 3: Induced expression of recombinase
[0030] The engineered bacterium constructed in Example 2 was inoculated into LB liquid medium containing 50 μg / mL kanamycin, cultivated overnight at 37° C., and then inoculated into 50 μg / mL kanamycin containing 50 μg / mL kanamycin with 1% inoculum size (v / v). In the LB liquid medium, 37 ℃, 150rpm cultivated to the cell concentration OD 600 To about 0.6, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 8000rpm for 10min at 4°C, and collect wet bacterial cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com