Preparation method of nickel-iron hydrotalcite water-oxidation electrode loaded on nitrogen-doped graphite foam
A graphite foam, nickel molten iron technology, applied in electrodes, electrode shape/type, electrolysis process, etc., can solve the problems of poor controllability of oxygen evolution reaction, inability to prepare large-area working electrodes, etc., to achieve mass production and save reactions Preparation cost, good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
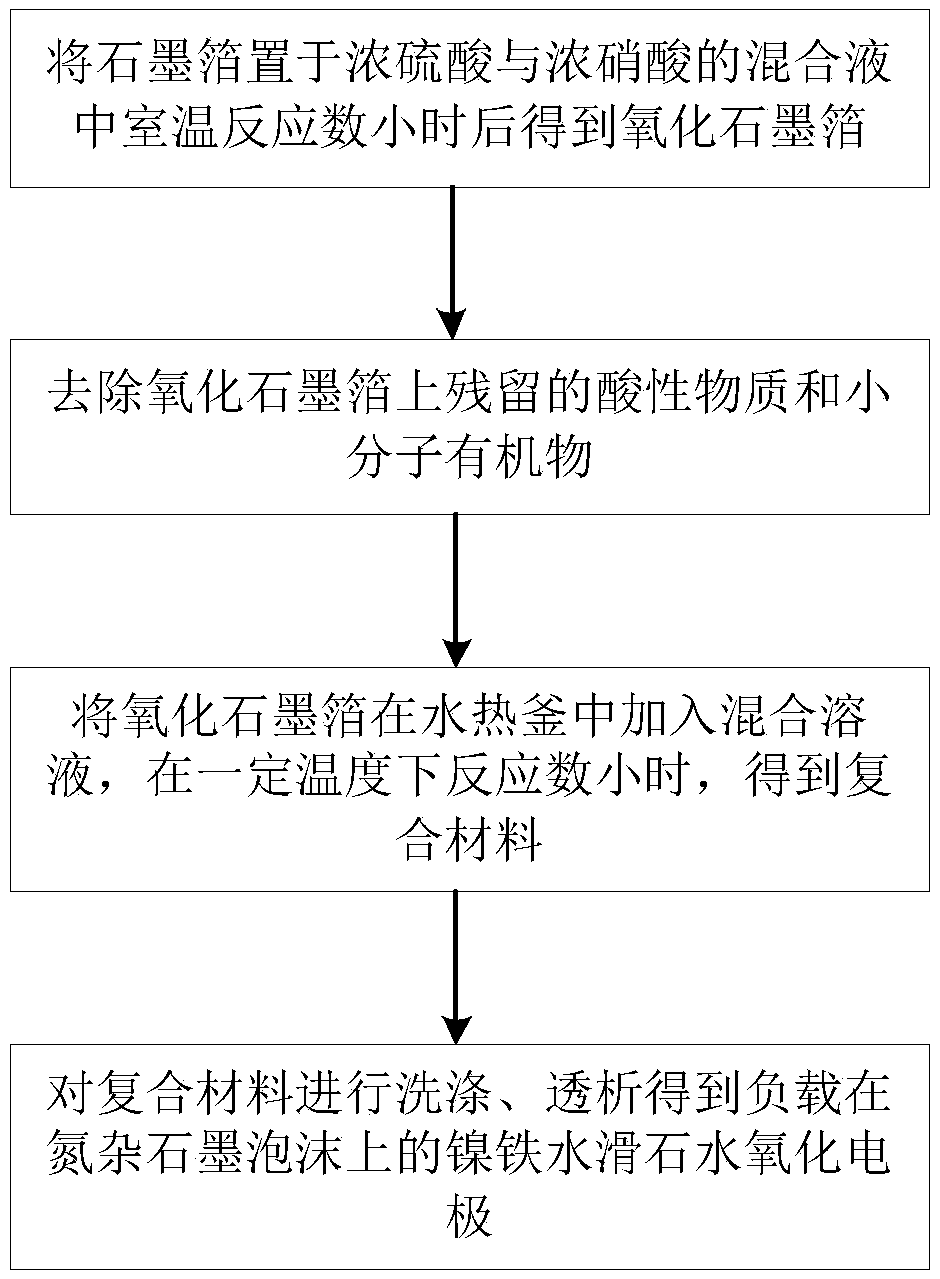
[0021] A preparation method of nickel-iron hydrotalcite water oxidation electrode supported on nitrogen-assorted graphite foam, comprising the steps of:
[0022] (S1) placing the required graphite foil in a mixed solution of concentrated sulfuric acid and concentrated nitric acid with a volume ratio of 3:1, and oxidizing at room temperature for 2-12 hours to obtain a loose and porous graphite oxide foil;
[0023] (S2) placing the graphite oxide foil in deionized water, and dialyzing to remove acidic substances and small molecular organics remaining on the graphite oxide foil;
[0024] (S3) Put the graphite oxide foil cleaned in (S2) in a hydrothermal kettle, add urea with a concentration of 0.1-2mol / L, and soluble divalent nickel salt nickel sulfate and nitric acid with a concentration of 10-200mmol / L Nickel or nickel chloride, a mixed solution of soluble ferric salt ferric sulfate, ferric nitrate or ferric chloride with a concentration of 2-100mmol / L, and hydrothermal reactio...
Embodiment 1
[0028] Cut the 200μm thick graphite foil into 4×2cm, and oxidize it in the mixed acid system of concentrated sulfuric acid:concentrated nitric acid=3:1 at room temperature for 6h to obtain graphite oxide foil, and dialyze the prepared graphite oxide foil in deionized water to remove the residual acidic substances and small molecular organic matter, put graphite oxide foil in a hydrothermal kettle, add 0.2mol / L urea, 20mmol / L soluble nickel salt, 5mmol / L soluble iron salt mixed solution, and react under 180°C for 12 hours. After washing with deionized water and dialysis, a nickel-iron hydrotalcite water oxidation electrode with the same size as graphite foil and uniformly loaded on azagraphite foam was obtained.
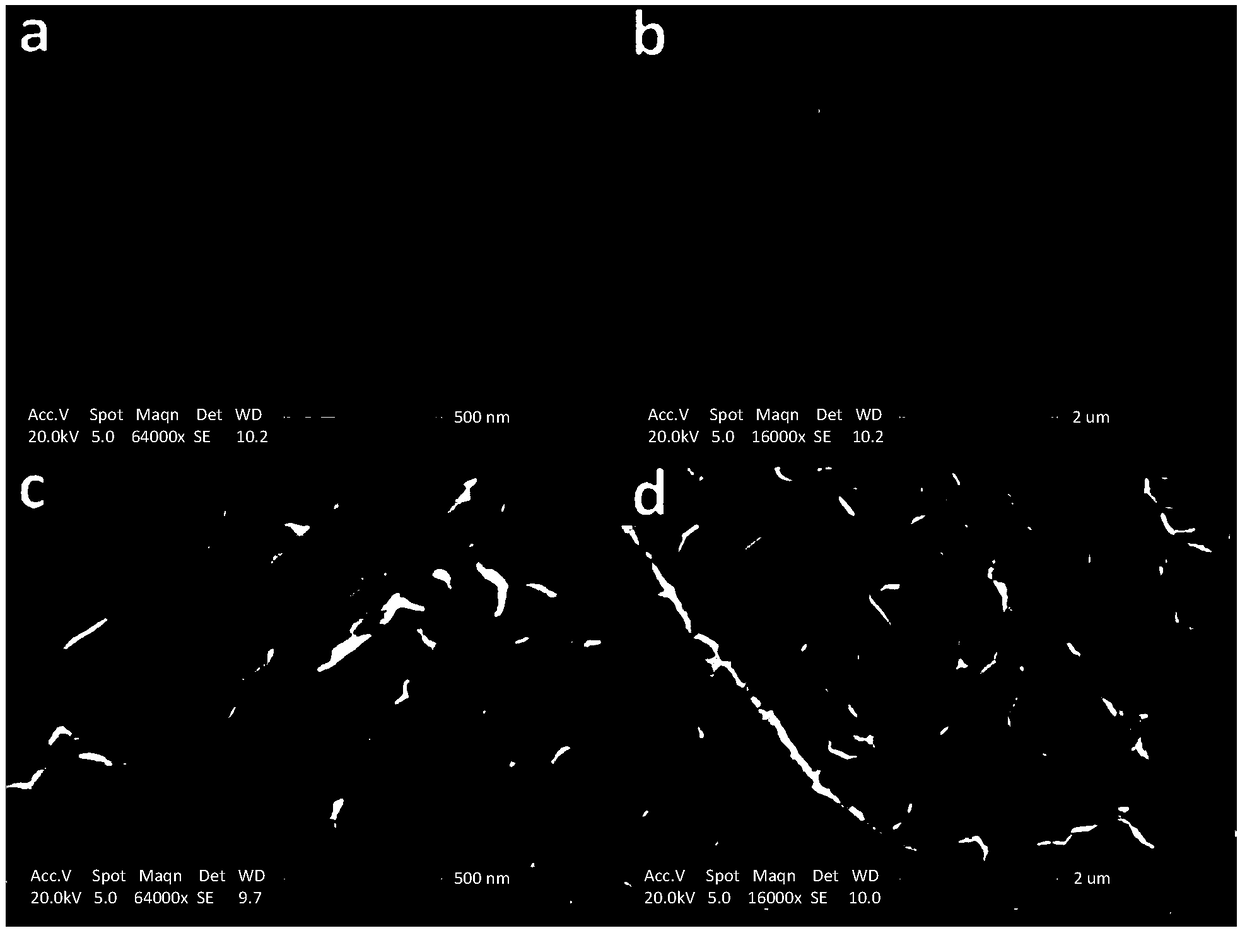
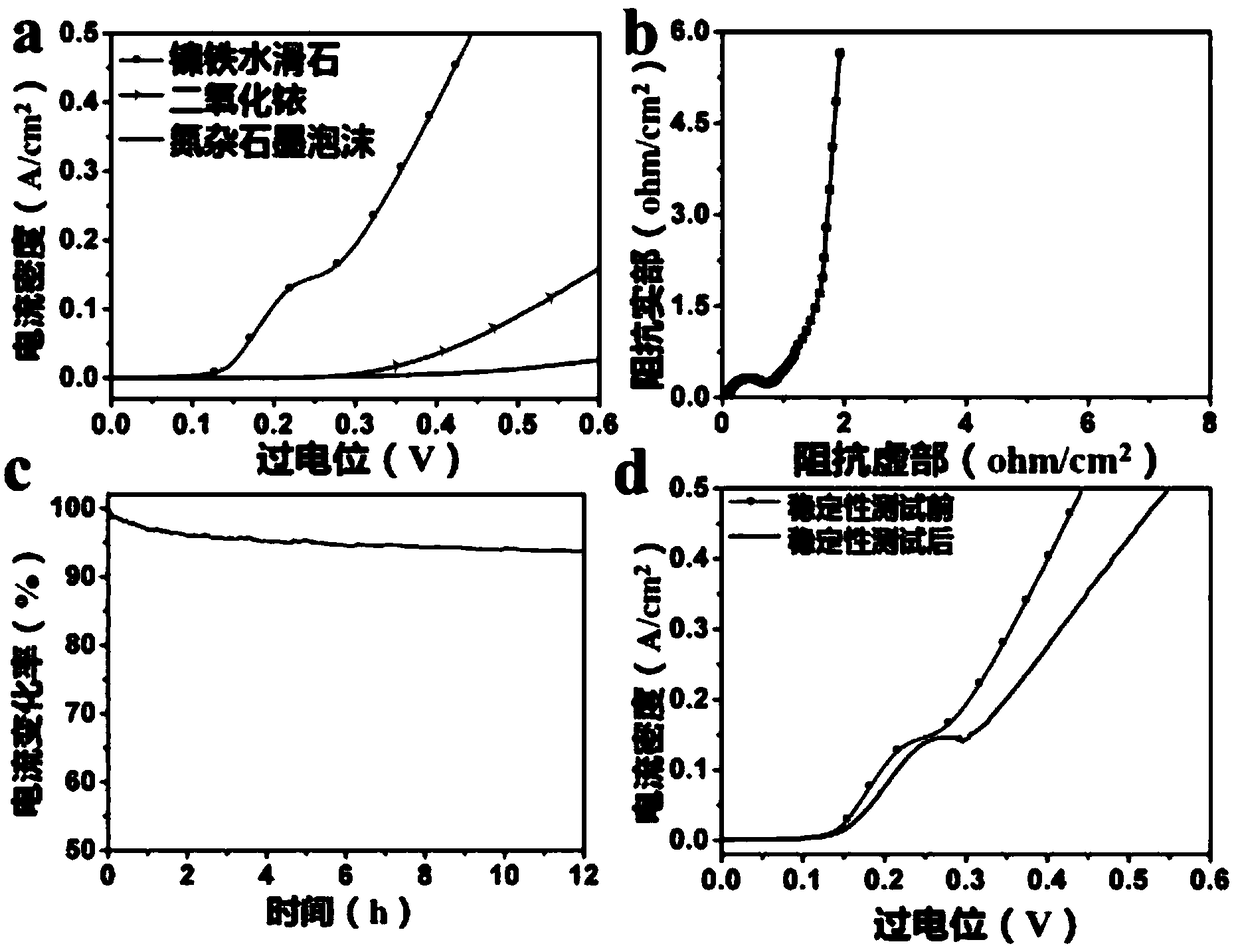
[0029] Depend on figure 2 In (a, b), it can be seen that the surface of the nitrogen graphite foam still maintains the wrinkled structure of the graphite surface after the hydrothermal reaction, and the size distribution of the nickel-iron hydrotalcite nanostructure ...
Embodiment 2
[0031] Cut the 500 μm thick graphite foil into 2×1cm, oxidize it in the mixed acid system of concentrated sulfuric acid:concentrated nitric acid=3:1 for 12h at room temperature to obtain graphite oxide foil, place the prepared graphite oxide foil in deionized water and dialyze to remove residual acidic substances and For small molecular organic matter, put the obtained graphite oxide foil in a hydrothermal kettle, add a mixed solution of 0.4mol / L urea, 40mmol / L soluble nickel salt, and 10mmol / L soluble iron salt, and conduct a hydrothermal reaction at 180°C for 12h , after cleaning and dialysis with deionized water, a nickel-iron hydrotalcite water oxidation electrode with the same size as graphite foil and uniformly loaded on azagraphite foam was obtained.
[0032] Depend on figure 2 (c, d) It can be seen that after increasing the concentration of nickel salt and iron salt, the surface wrinkle structure of azagraphite foam remains unchanged, and the nanostructure size of nic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com