A-site layered perovskite type electrode material and preparation method thereof
An electrode material and perovskite-type technology, which is applied in the field of A-site layered perovskite-type electrode materials and its preparation, can solve the problems of fuel gas and oxidant catalytic activity that are not very ideal, so as to improve redox catalytic activity, The effect of increasing the conductivity and increasing the concentration of oxygen vacancies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
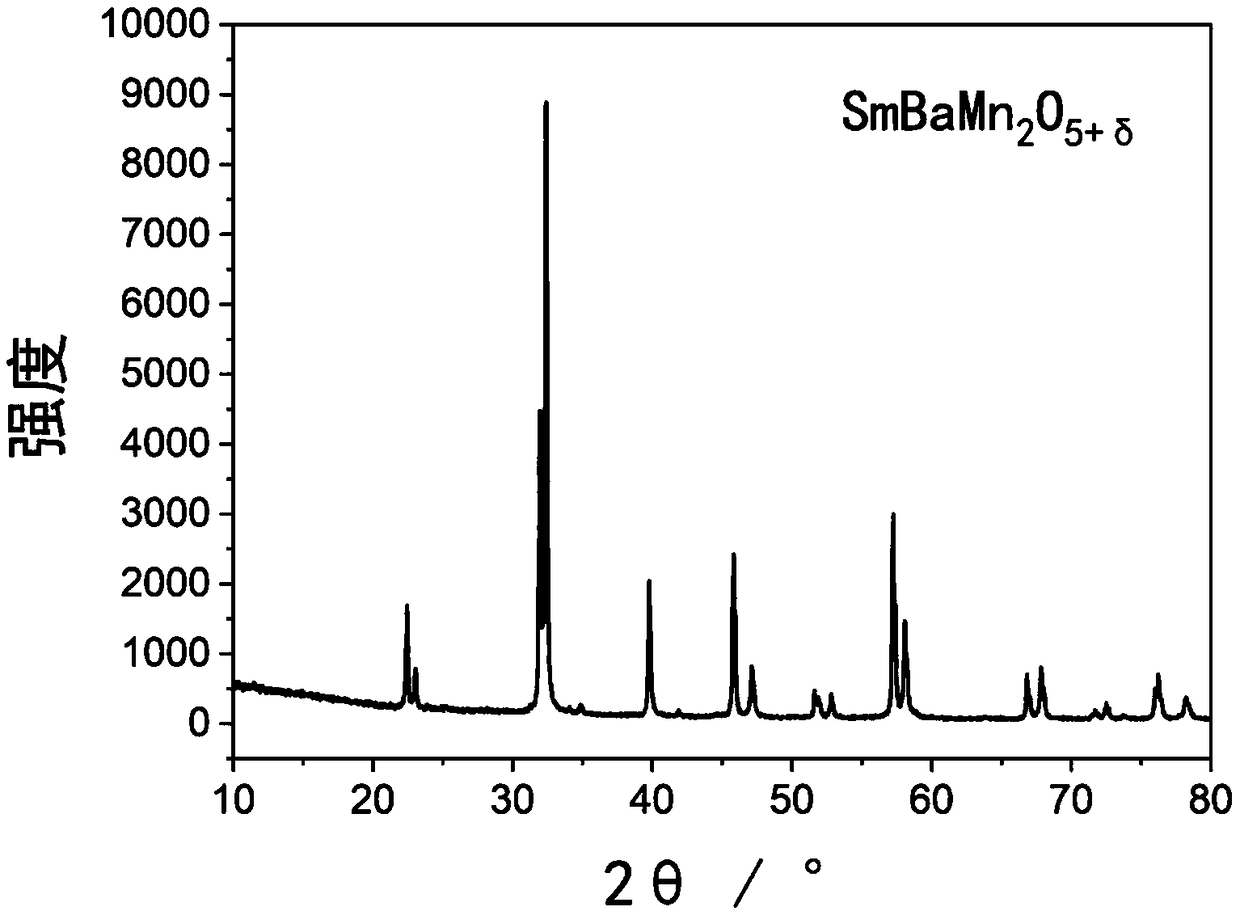
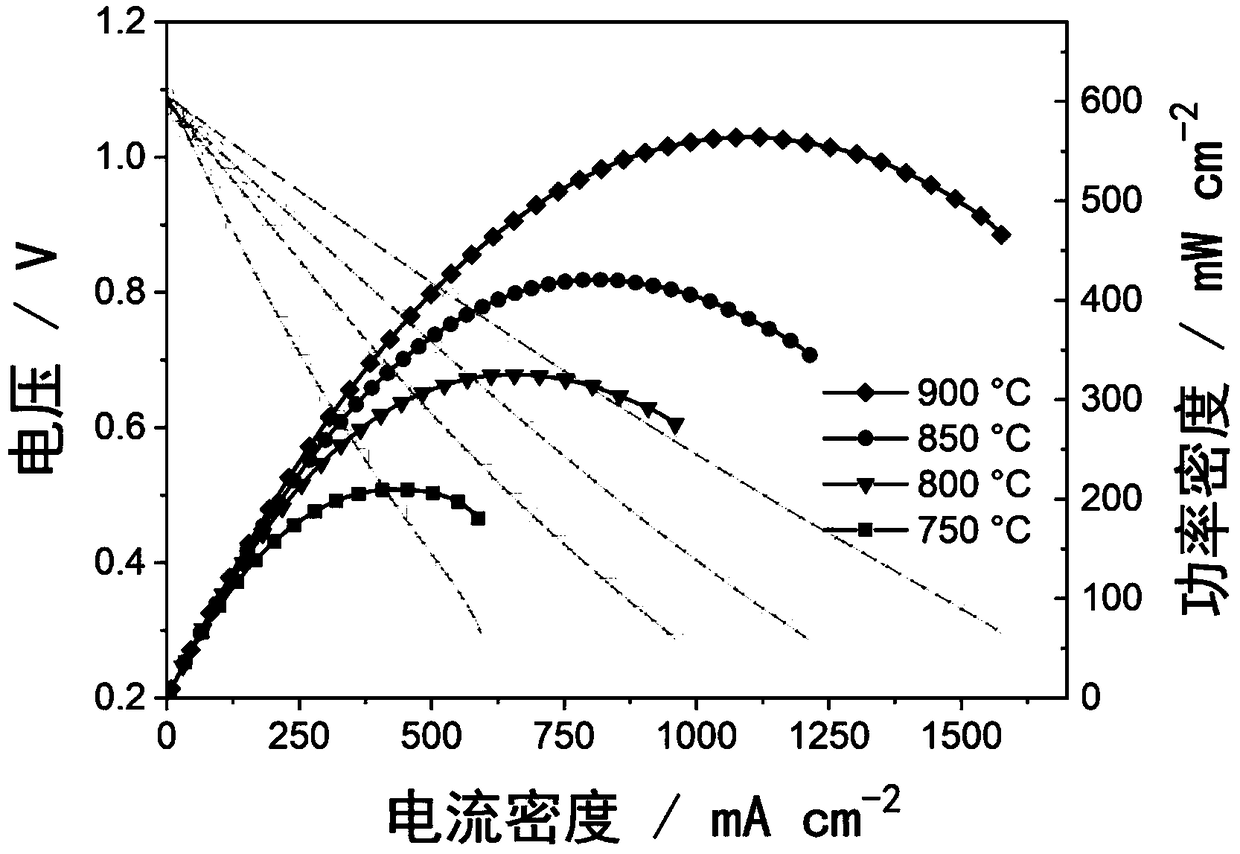
[0032] Synthesis of LnBaMn by Citric Acid-Combustion Method 2-x m x o 5+δ (Ln=Sm, M=Mn) Dense sample. According to SmBaMn 2 o 5+δ Stoichiometric ratio preparation, the Sm 2 o 3 , Ba(NO 3 ) 2 、C 4 h 6 MnO 4 4H 2 Dissolve O in deionized water respectively, add citric acid at a ratio of 1:2 to metal ions and citric acid, add EDTA at a ratio of 1:1 to metal ions and EDTA and stir continuously to form a uniform solution, and add ammonia water Adjust the pH value of the solution to 3. Then obtain a homogeneous sol in a water bath at 50°C, place the sol in an oven at 100°C to obtain a dry gel, and then heat it at 300°C until it spontaneously burns to form a very fluffy SmBaMn 2 o 5+δ Precursor powder. Grind the powder, put it into a high-temperature furnace for roasting, the temperature is 800°C, and the holding time is 12 hours, so that the organic matter in it is fully decomposed. The above powders were ground and placed in a tube furnace at H 2 Treat in a mixed ga...
Embodiment 2
[0034] Synthesis of LnBaMn by Citric Acid-Combustion Method 2-x m x o 5+δ (Ln=Nd, M=Fe, x=0.2) Dense sample. According to NdBaMn 1.8 Fe 0.2 o 5+δ Stoichiometric ratio preparation, the Nd 2 o 3 , Ba(NO 3 ) 2 、C 4 h 6 MnO 4 4H 2 O and Fe(NO 3 ) 3 9H 2 Dissolve O in deionized water respectively, add citric acid at a ratio of 1:2 to metal ions and citric acid, add EDTA at a ratio of 1:1 to metal ions and EDTA and stir continuously to form a uniform solution, and use ammonia water Adjust the pH of the solution to 8. Then obtain a uniform sol in a water bath at 100°C, heat the sol in an oven at 50°C to obtain a dry gel, and then heat it at 200°C until it spontaneously burns to form a very fluffy NdBaMn 1.8 Fe 0.2 o 5+δ Precursor powder. Grind the powder, put it into a high-temperature furnace for roasting, the temperature is 1200°C, and the holding time is 4 hours, so that the organic matter in it can be fully decomposed. The above powders were ground and placed...
Embodiment 3
[0036] Synthesis of LnBaMn by Citric Acid-Combustion Method 2-x m x o 5+δ (Ln=Gd, M=Co, x=0.5) electrode powder. According to GdBaMn 1.5 co 0.5 o 5+δ Stoichiometric preparation, the Gd 2 o 3 , Ba(NO 3 ) 2 、C 4 h 6 MnO 4 4H 2 O, Co(NO 3 ) 2 ·6H 2 Dissolve O in deionized water respectively, add citric acid at a ratio of 1:2 to metal ions and citric acid, add EDTA at a ratio of 1:1 to metal ions and EDTA and stir continuously to form a uniform solution, and use ammonia water Adjust the pH of the solution to 6. Then obtain a uniform sol in a water bath at 70°C, place the sol in an oven at 80°C to obtain a dry gel, and then heat it at 240°C until it self-combusts to form a very fluffy GdBaMn 1.5 co 0.5 o 5+δ Precursor powder. Grind the powder, put it into a high-temperature furnace for roasting, the temperature is 1100°C, and the holding time is 10h, so that the organic matter in it can be fully decomposed. The above powders were ground and placed in a tube fur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com