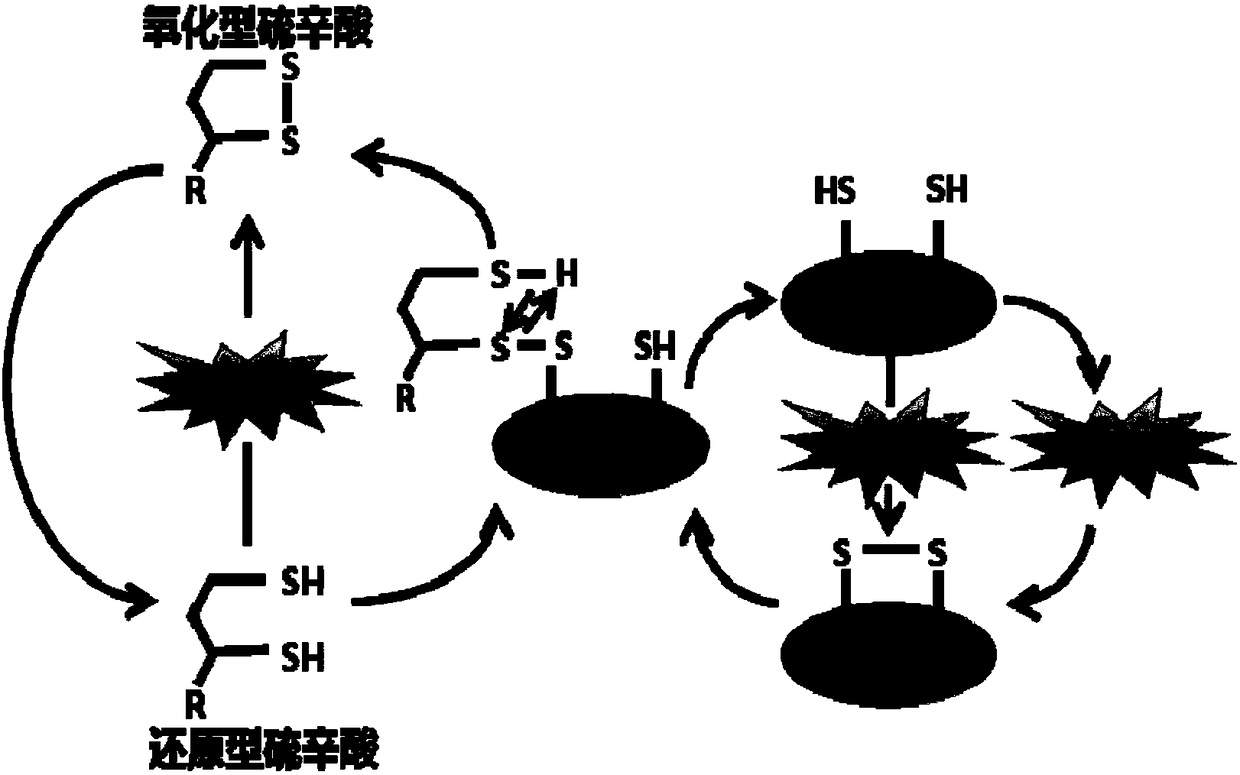
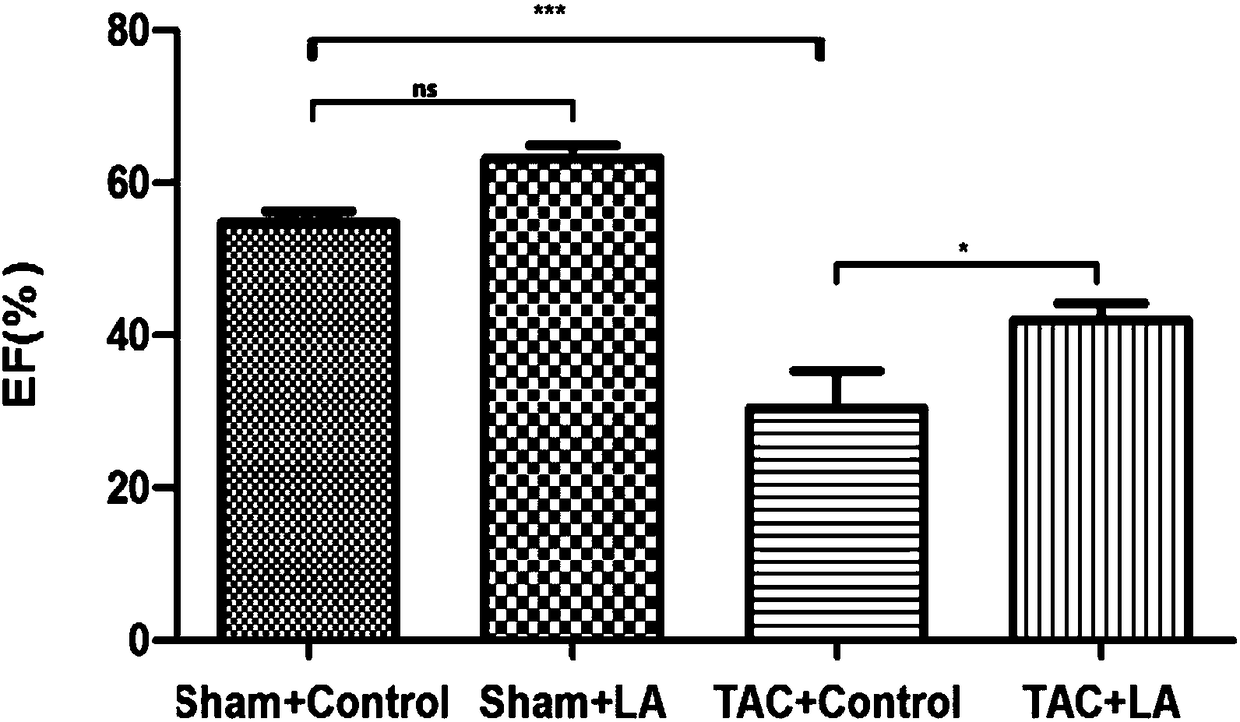
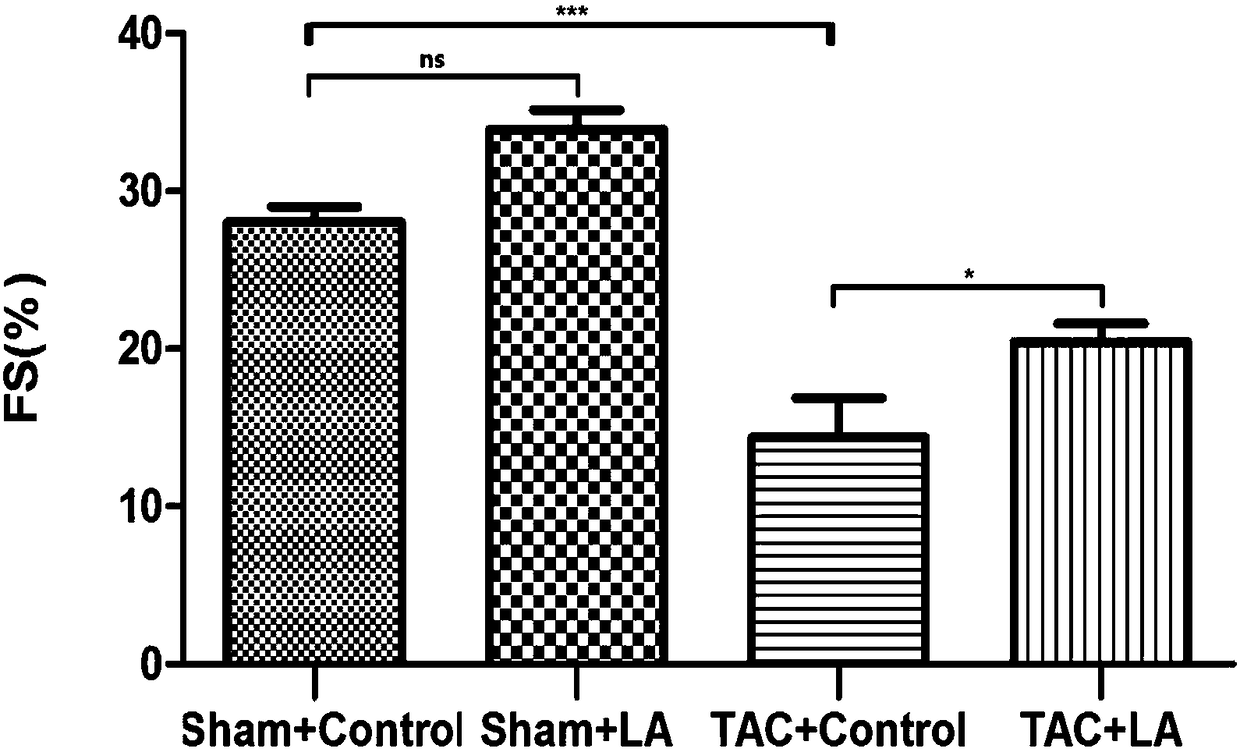
Use of lipoic acid in preparation of pharmaceutical composition for treatment on pressure-loaded myocardial injury
A technology for myocardial injury and lipoic acid, which is applied in the field of medicine, can solve the problems of unreported lipoic acid, many medicinal materials, and inconvenient use, and achieve the effects of protecting myocardial injury, improving energy metabolism, and inhibiting oxidative stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 capsule (one)
[0037] The preparation method of lipoic acid capsules is as follows:
[0038] Lipoic acid was purchased from Jiangsu Wanchuan Medical and Health Industry Group Co., Ltd.
[0039] (1) get 100g lipoic acid, micronize, cross 200 mesh sieves, get lipoic acid fine powder;
[0040] (2) Put 50g of Poloxamer 237 in a suitable container, put it in a water bath at 49°C-50°C and heat it to a molten liquid state, add 100g of lipoic acid fine powder, and stir quickly and fully at 49°C-50°C until the mixture is uniform , stand still to remove air bubbles, spread the mixture into a thin layer and put it in a -5°C refrigerator for rapid cooling. After the mixture is completely solidified, take it out and pulverize it, and dry it in a vacuum dryer at 35°C for 24 hours, and pulverize it through a 100-mesh sieve to obtain sulfur Caprylic Acid Solid Dispersion.
[0041](3) Add 82g of microcrystalline cellulose, 10g of lactose, 8g of sodium...
Embodiment 2
[0042] The preparation of embodiment 2 intravenous injections (two)
[0043] The preparation method of lipoic acid intravenous injection is as follows:
[0044] Lipoic acid was purchased from Jiangsu Wanchuan Medical and Health Industry Group Co., Ltd.
[0045] (1) Place co-solvent ethylenediamine in water for injection cooled to room temperature, and mix well;
[0046] (2) Measure lipoic acid and co-solvent ethylenediamine according to the mass ratio of lipoic acid and co-solvent ethylenediamine as 1:0.23, place lipoic acid in co-solvent ethylenediamine solution, and stir until completely dissolved;
[0047] (3) Weigh hydroxypropyl-β-cyclodextrin according to the mass ratio of lipoic acid to hydroxypropyl-β-cyclodextrin as 1:2.5, and add hydroxypropyl-β-cyclodextrin to step (2) In the prepared solution, stir until completely dissolved;
[0048] (4) Add water for injection to the full amount;
[0049] (5) potting the medicinal solution in a brown ampoule;
[0050] (6) Ste...
Embodiment 3
[0051] Embodiment 3 animal experiments
[0052] 1. Experimental method
[0053] 1. Preparation of experimental animals and animal models
[0054] The mouse model preparation method of operation group (TAC group): SPF grade male C57BL / 6 mice (purchased from Shanghai Jiesijie Experimental Animal Co., Ltd.), aged 8-10 weeks, weighing 20-25g, were selected for tracheal intubation. Put the mouse in an anesthesia airtight box, and after the muscle strength disappears, quickly lie supine and fix it on the operation board; aim the cold light source at the neck of the mouse, and gently pull out the tongue of the mouse with tweezers , under the irradiation of a cold light source, you can see a tracheal opening with a strong refraction that is consistent with the respiratory rate. Quickly insert the endotracheal tube into the airway. Connect to the anesthesia ventilator. If the intubation is successful, you can see that the mouse’s chest cavity rises and falls with the frequency of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com