Dual-valve multi-stimulation-responsive drug carrier constructed on basis of mesoporous silicon/cyclodextrin/zinc oxide quantum dots and preparation method thereof
A zinc oxide and mesoporous silicon technology is applied in the field of "dual valve" multi-stimulus-responsive drug carrier and its preparation, and achieves the effects of high efficiency, reducing premature release and improving drug utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
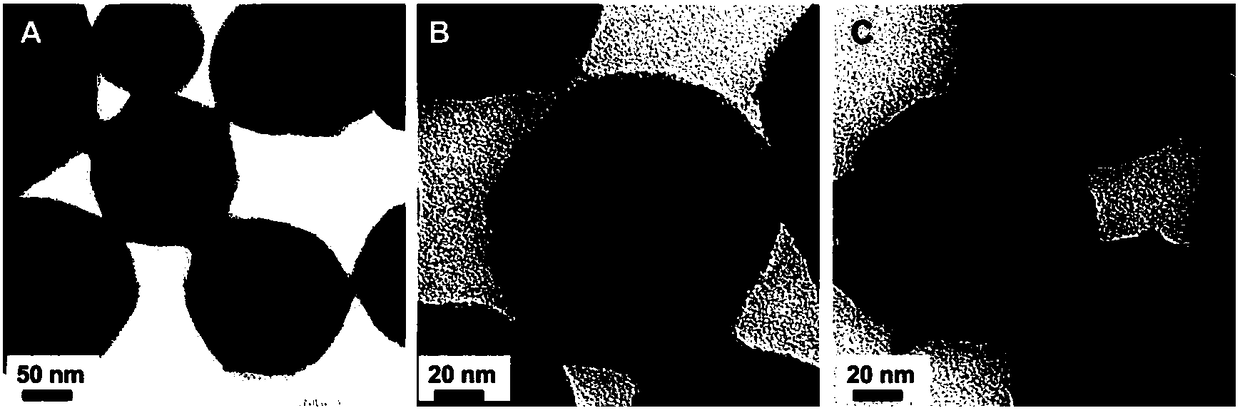
[0041] The preparation steps of MSN are as follows: first dissolve NaOH (0.28g) and CTAB (1g) in 480mL of secondary water and raise the temperature to 80°C, then slowly add TEOS (5mL) into the system dropwise, and continue stirring for 2h to produce white precipitate. After the reaction, the product was centrifuged (9500r / min×10min), washed with secondary water and methanol several times, and vacuum-dried at 50°C to obtain the MSN containing the template CTAB (CTAB@MSN). In order to remove the template CTAB, the obtained CTAB@MSN (0.5g) needs to be refluxed in methanol (160mL) containing 9mL of concentrated hydrochloric acid for 48h, then washed and centrifuged several times with secondary water and methanol, and dried under vacuum at 50°C to obtain MSN .
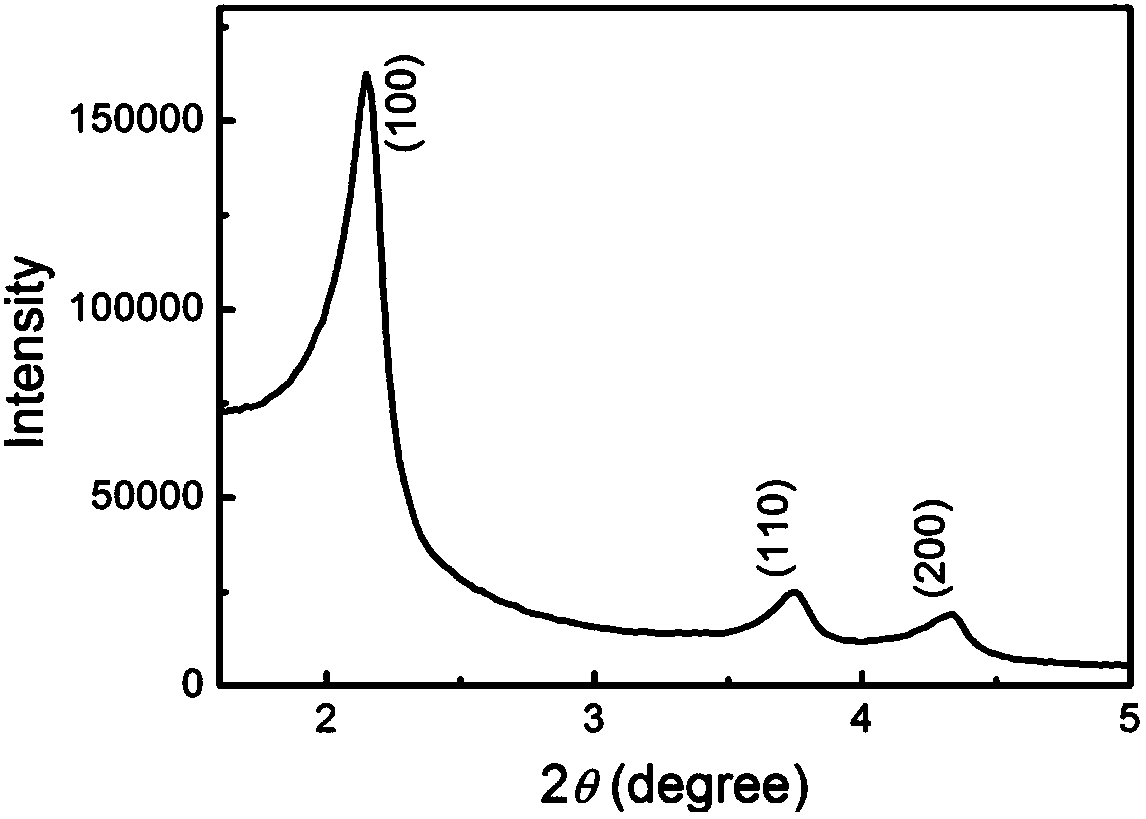
[0042] Such as figure 2 As shown in the small-angle X-ray diffraction pattern, it can be seen that the MSN channels are arranged in a hexagonal shape, with three characteristic small-angle diffraction peaks corresponding...
Embodiment 1
[0054] A composite nano drug carrier system, comprising the steps of:
[0055] 1. Preparation of aminated zinc oxide quantum dots (ZnO-NH 2 QDs)
[0056] Disperse ZnO QDs in 20ml of anhydrous N'N-dimethylformamide (DMF), add 100ul of triaminopropyltriethoxysilane (APTES), react at 120°C for 20min, and then wash with DMF for several minutes times, vacuum drying at 50 degrees to obtain white ZnO-NH 2 QDs.
[0057] 2. Preparation of drug carrier DOX@MSN-N-ZnO blocked by zinc oxide quantum dots
[0058] Disperse 0.2g of MSN-COOH in 50ml of dichloromethane, add 50mg of doxorubicin (DOX), stir at room temperature for 24 hours in the dark, add 8.4g of DCC, 0.64g of DMAP after activation at room temperature for 8 hours, add 0.5g of ZnO -NH 2 After reacting for 48 hours, the QDs were washed twice with methanol and twice with water, and freeze-dried to obtain DOX@MSN-N-ZnO.
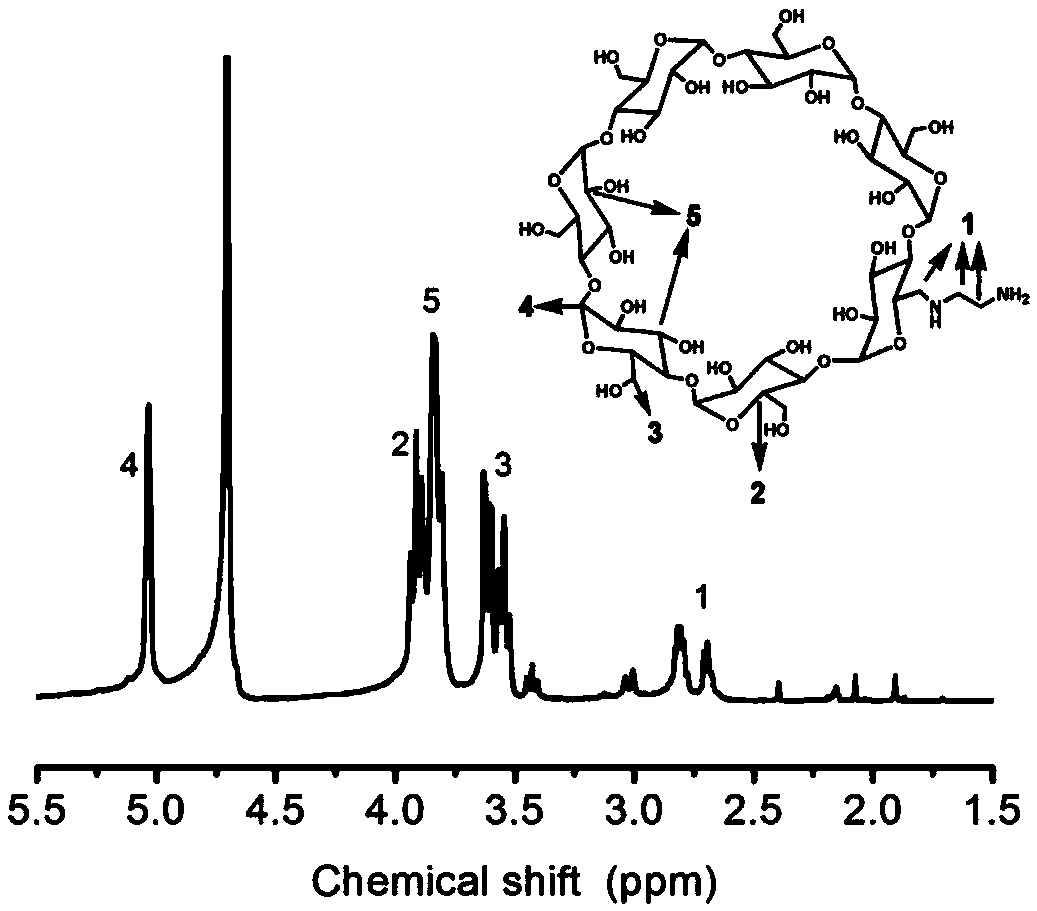
[0059] 3. Preparation of anhydrided cyclodextrin β-CD-cit
[0060] Take 1gβ-CD-NH 2 , dissolved in 100ml...
Embodiment 2
[0074] A composite nano drug carrier system, comprising the steps of:
[0075] 1. Preparation of aminated zinc oxide quantum dots (ZnO-NH 2 QDs)
[0076] Disperse ZnO QDs in 20ml of anhydrous N'N-dimethylformamide (DMF), add 100ul of triaminopropyltriethoxysilane (APTES), react at 120°C for 20min, and then wash with DMF for several minutes times, vacuum drying at 50 degrees to obtain white ZnO-NH 2 QDs.
[0077] 2. Preparation of drug carrier DOX@MSN-N-ZnO blocked by zinc oxide quantum dots
[0078] Disperse 0.2g of MSN-COOH in 50ml of dichloromethane, add 100mg of doxorubicin (DOX), stir at room temperature for 24 hours in the dark, add 8.4g of DCC, 0.64g of DMAP after activation at room temperature for 8 hours, add 0.5g of ZnO -NH 2 After reacting for 48 hours, the QDs were washed twice with methanol and twice with water, and freeze-dried to obtain DOX@MSN-N-ZnO.
[0079] 3. Preparation of anhydrided cyclodextrin β-CD-cit
[0080] Take 1gβ-CD-NH 2 , dissolved in 100m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ll | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com