Terphenyl compound as well as preparation method and application thereof
A technology of terphenyls and compounds, which is applied in the field of microbial engineering, can solve the problems of underutilization, etc., and achieve the effect of short cycle and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0039] (1) The Streptomyces chrysalis 1510-2 described in the present invention is isolated from the body of the chrysalis chrysalis through strict surface disinfection.
[0040] (2) Streptomyces chrysalis 1510-2 of the present invention is in the form of air silk powder on the colonies of Gao's No. 1 solid medium, taupe-gray. It was initially identified as Streptomyces.
[0041](3) Streptomyces chrysalis 1510-2 of the present invention, on Gao's No. 1 agar medium, the air filaments are off-white, the base filaments are yellowish brown, and the spore filaments are long and straight, forming clusters. The spores are nearly spherical to ellipsoid, with a smooth surface. identified as Streptomyces.
[0042] (4) Streptomyces chrysalis 1510-2 described in the present invention has been preserved in the China Type Culture Collection Center (Address: Wuhan Luojiashan Wuhan University) on March 11, 2018, and the preservation number is CCTCC NO :M 2018111. .
Embodiment 1
[0044] Isolation and Purification of Streptomyces 1510-2
[0045] The Streptomyces used in the present invention is a strain of symbiotic actinomycetes isolated from the body of Sclerex chinensis from the suburbs of Dezhou City, Shandong Province in 2015, and the strain number is N1510-2.
[0046] Under aseptic conditions, the body surface of the yellow stalk wall wasp was sterilized with 70% alcohol, the bee was placed in a sterile mortar and a small amount of sterile water was added for grinding, and the grinding solution was diluted to 10- 1, 10-2, 10-3, respectively take 100 microliters of each gradient dilution solution in Gaucher’s medium and YMS medium, and place them in a 37°C incubator for cultivation. After the colonies grow, pick the spores for purification and culture , to obtain endosymbiontic actinomycetes of Sclerex chinensis.
Embodiment 2
[0047] Example 2: Identification of Streptomyces 1510-2
[0048] (1) Morphological identification:
[0049] The surface of the colony of Streptomyces 1510-2 on Gao's No. 1 medium is off-white, and the colony is round like figure 1 shown. Optical microscope inspection showed that the spore filaments were long and straight, and clustered. The spores are nearly spherical to oval, with a smooth surface, and were initially identified as Streptomyces such as figure 2 shown.
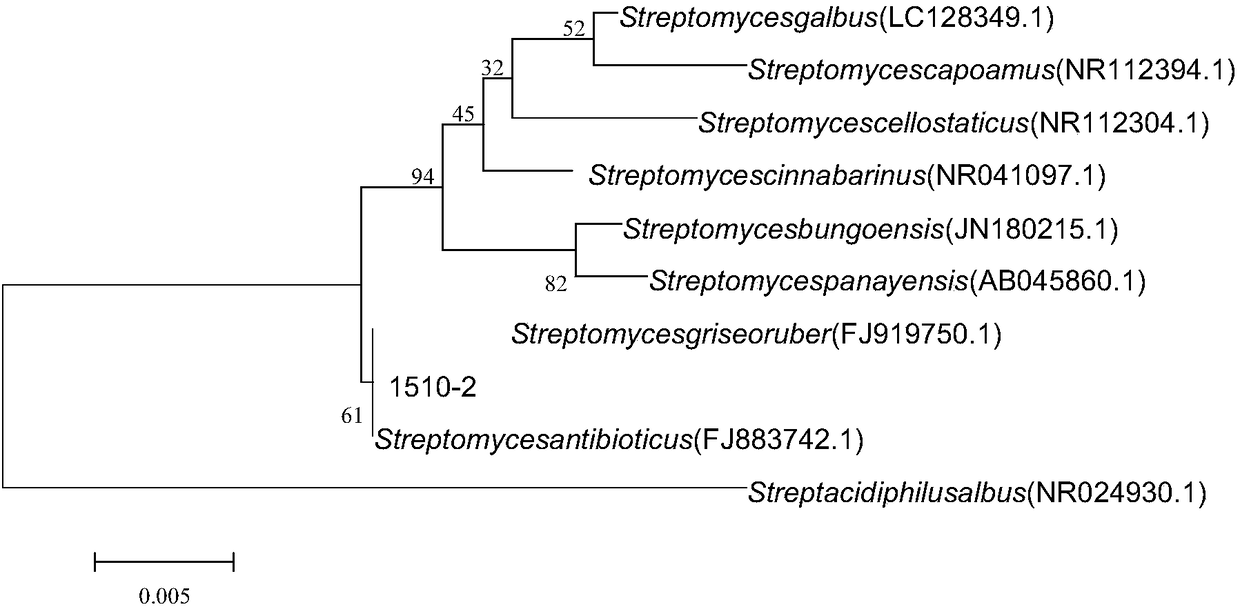
[0050] (2) Molecular identification: DNA was extracted from the mycelium according to the instructions of the Sangong Bacteria Genomic DNA Rapid Extraction Kit, and the forward primer was Fd2: 5'-GAGTTTGATCATGGCTCAG-3, and the reverse primer 16Sr: 5'-TTGCGGGACTTAACCCAACAT-3 16S rDNA sequence was amplified by PCR, and the PCR product was purified and sent to Huada Company for sequencing. The sequencing results were compared and analyzed by BLAST, similar sequences were selected, and the phylogenetic tree wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com