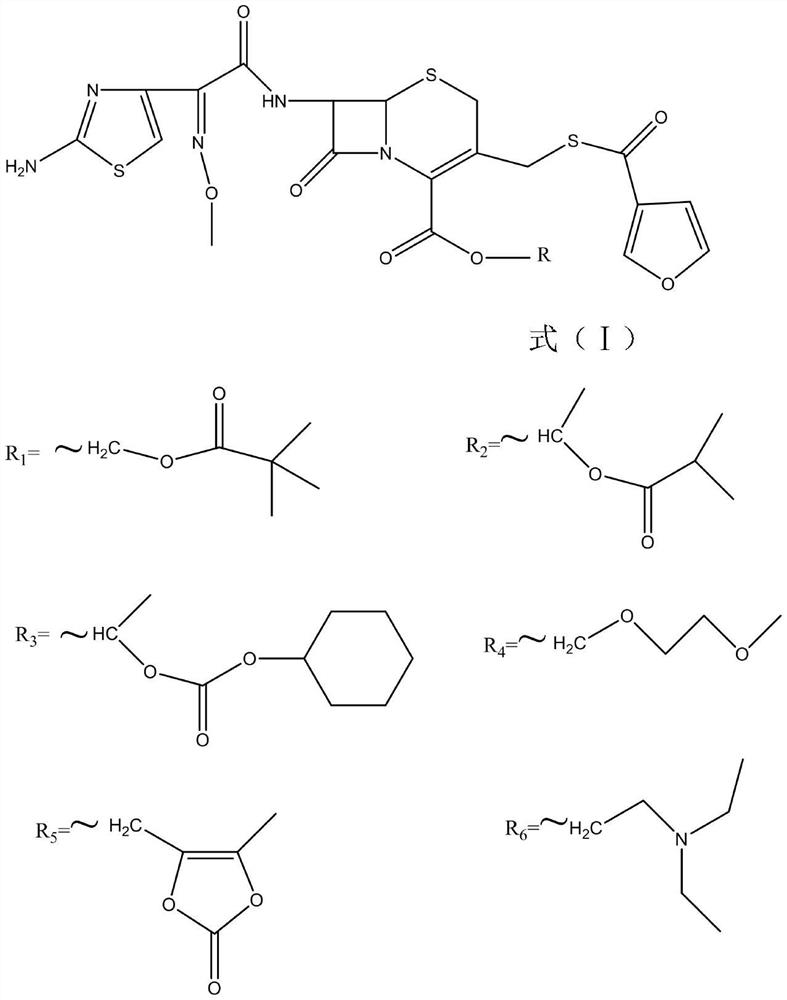
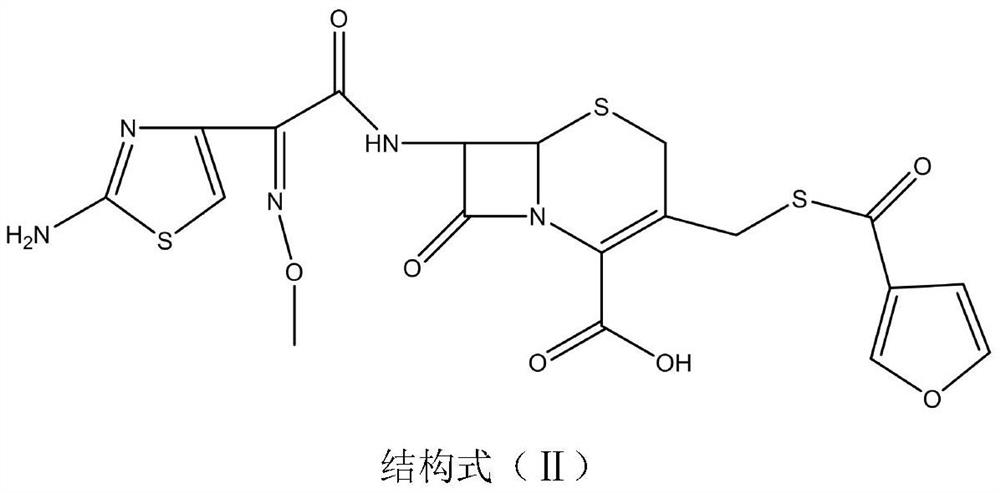
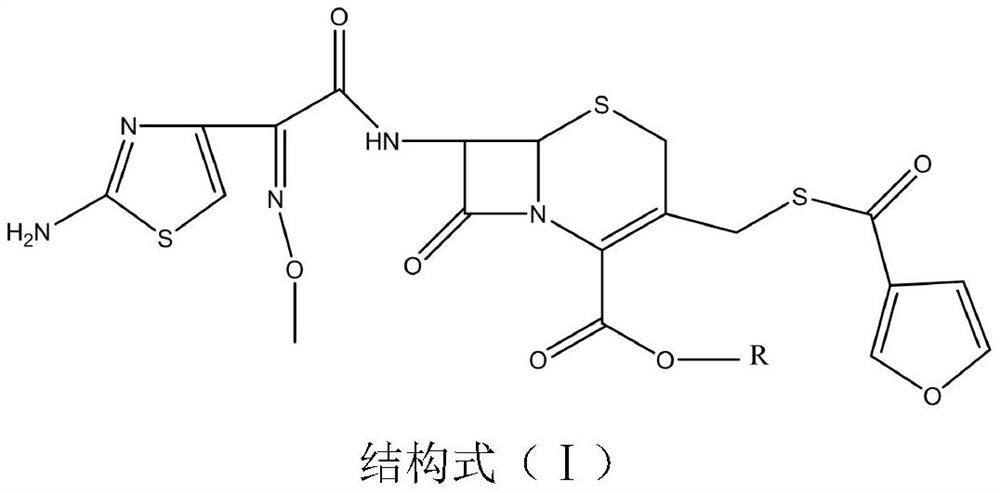
Preparation method of ceftiofur axetil compound or its halogen salt
A compound and thifurate technology are applied in the field of preparation of ceftiofur axetil compounds or their halogen salts, which can solve the problems of cumbersome preparation process, low reaction rate, environmental pollution and the like, and achieve green environmental protection and bioavailability of the preparation process. Low, increase the effect of specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh 21g (0.04mom) of ceftiofur (self-made 20170816), add it to a 500mL three-necked flask, add 40mL of methanol, add 5.5g (0.04mol) of potassium carbonate and stir to dissolve, add 50g of D201 resin, cool to 10°C, add 40ml of ethyl acetate, then add 12g (0.05mol) iodomethyl pivalate for 90 minutes of ultrasonic reaction at 35°C. Filter, wash the filter cake with 100mL of ethyl acetate, rotary evaporate the filtrate to 30mL at 40°C, add 50ml of isopropanol to dilute, Add 150mL of isopropyl ether dropwise for no less than 30min, grow crystals at room temperature for 1h, filter, and vacuum dry at 40°C for 8h to obtain 18.2g of off-white powder with a purity of 97%.
Embodiment 2
[0053] Weigh 21g (0.04mom) of ceftiofur (self-made 20170816), add it to a 500mL three-necked flask, add 40mL of methanol, then add 5.5g (0.04mol) of potassium carbonate and stir to dissolve it, add 50g of D201 resin, cool down to 10°C, Add 40 ml of ethyl acetate, add 12 g (0.05 mol) of iodoethyl isopropyl carbonate and react ultrasonically at 35 ° C for 90 minutes. Filter, wash the filter cake with ethyl acetate, and rotary evaporate the filtrate to 30 mL at 40 ° C, add 50 ml of isopropanol to dilute , add 150mL isopropyl ether dropwise, the dropping time is not less than 30min, grow crystals at room temperature for 1h, filter, and vacuum dry at 40°C for 8h to obtain 17.3g of off-white powder with a purity of 96.5%.
Embodiment 3
[0055]Weigh 21g (0.04mom) of ceftiofur (self-made 20170816), add it to a 500mL three-necked flask, add 40mL of methanol, then add 5.5g (0.04mol) of potassium carbonate and stir to dissolve it, add 50g of D201 resin, cool down to 10°C, Add 40ml of ethyl acetate, add 15g (0.05mol) of 1-iodoethylcyclohexyl carbonate and react ultrasonically at 35°C for 90 minutes. Filter, wash the filter cake with ethyl acetate, rotary evaporate the filtrate to 30mL at 40°C, add 50ml of isopropanol Dilute, add 150mL isopropyl ether dropwise, dropwise for not less than 30min, grow crystals at room temperature for 1h, filter, and vacuum dry at 40°C for 8h to obtain 20.2g of off-white powder with a purity of 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com