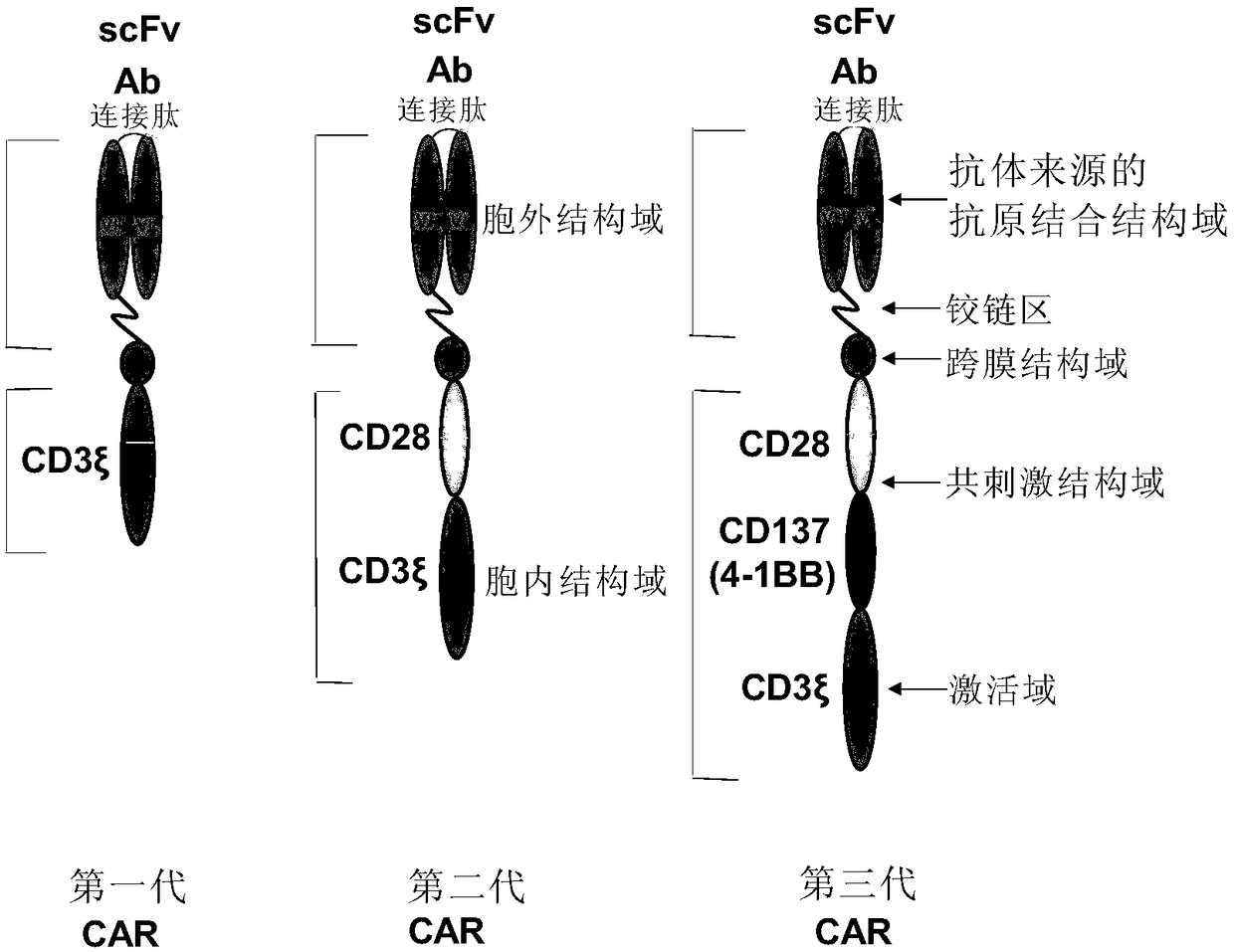
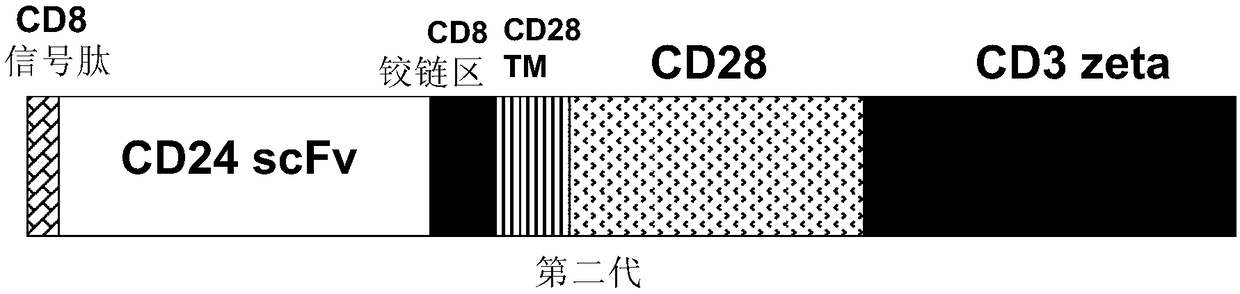
CD24 specific antibody and anti-CD24-CAR-T cell
A CD24, antibody technology, applied in the field of adoptive immune gene therapy of tumors, can solve the problems of low safety and high recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] Antibody preparation
[0114] Any method suitable for producing monoclonal antibodies can be used to produce anti-CD24 antibodies of the invention. For example, animals can be immunized with linked or naturally occurring CD24 homodimers or fragments thereof. Suitable immunization methods may be used, including adjuvants, immunostimulants, repeated booster immunizations, one or more routes may be used.
[0115] Any suitable form of CD24 can be used as an immunogen (antigen) for producing non-human antibodies specific to CD24 and screening the biological activity of the antibodies. The priming immunogen can be full-length mature human CD24, including native homodimers, or single / multiple epitope-containing peptides. Immunogens can be used alone or in combination with one or more immunogenicity enhancers known in the art. Immunogens can be purified from natural sources, or produced in genetically modified cells. The DNA encoding the immunogen can be genomic or non-geno...
Embodiment 1
[0191] Example 1 Production of CAR lentivirus
[0192] Lentiviruses were prepared by the following steps:
[0193] Day 1:
[0194] 1. Place 5×10 6 HEK293FT cells were seeded into 100 mm diameter petri dishes;
[0195] Day 2:
[0196] 2. Check to make sure the cells are 70%-90% confluent;
[0197] 3. Prepare the transfection complex for each 100 mm diameter Petri dish as follows:
[0198] a. In 1.5ml tube A: dilute 2.5μg CAR (chimeric antigen receptor) DNA plasmid (plasmid) and 20μL lentiviral packaging mix (ALSTEM, catalog number VP100; see Appendix B3) to 0.5ml DMEM or Opti- In MEM serum-free medium, mix gently;
[0199] b. In 1.5ml tube B: Dilute 30μL Nanofect Transfection Reagent (ALSTEM, catalog number NF100) into 0.5ml DMEM or Opti-MEM serum-free medium, mix gently;
[0200] c. Add the NanoFect / DMEM in tube B to the DNA / DMEM solution (tube A), vortex for 5-10 seconds, and incubate the DMEM-plasmid-NanoFect mixture at room temperature for 15 minutes;
[0201] 4. Ad...
Embodiment 2
[0212] Example 2 lentiviral packaging system
[0213] Product Description
[0214] Product name: SuperLenti TM Lentivirus Packaging System
[0215] manual:
[0216] For the production of lentiviral particles, three components are generally required: 1) a lentiviral vector containing the foreign gene of interest, 2) a packaging vector containing all the necessary viral structural proteins, 3) expressing vesicular stomatitis virus (VSV) sugars Envelope carrier for protein (G). The third-generation lentiviral packaging system provides maximum biosafety because the lentiviral Rev gene is provided as a separate vector from other structural genes, further eliminating the possibility of reverse recombination of the vector into replication-competent virus particles. Third-generation lentiviral packaging mixes only support lentiviral expression vectors with chimeric 5’LTRs, where the HIV promoter is replaced by CMV or RSV, thus making it independent of TAT.
[0217] The SuperLent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com