Method for removing fluorine from sulfate solution
A sulfate and solution technology, applied in sulfate preparation, alkali metal sulfite/sulfate purification, alkali metal fluoride, etc., to achieve the effect of simple process, avoidance of fluorine-containing solid waste, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 5 m of zinc electrolyte solution with a pH of 5.2 3 , add calcium sulfate according to 4.5 times of the theoretical amount of fluorine converted into calcium fluoride, stir at room temperature for 2.5 hours to absorb and remove the fluorine, and filter to obtain the defluoridated liquid and fluorine-containing filter residue. The F content in the liquid after defluoridation is 13mg / L, which can be directly used for zinc electrolysis. The fluorine-containing filter residue is stirred into water at a solid-to-liquid ratio of 1:2g / mL, heated to 65°C, and potassium carbonate is slowly added to adjust the pH to 8.6. Continue to stir for 2.5h to make it completely transformed, and filter to obtain calcium carbonate filter cake and potassium fluoride solution. Then add sodium carbonate to the potassium fluoride solution according to 0.95 times of the theoretical amount converted into sodium fluoride, stir at 80°C for 1 hour, and filter to obtain sodium fluoride crystals a...
Embodiment 2
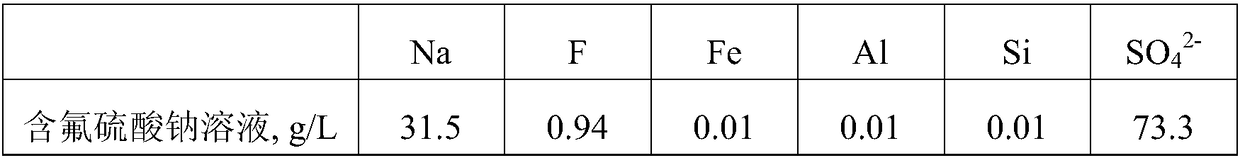
[0037]Take 1.5m of fluorine-containing sodium sulfate solution with a pH of 8.2 3 , add calcium sulfate according to 3.5 times the theoretical amount of fluorine converted into calcium fluoride, stir and ball mill at room temperature for 1 hour to absorb and remove the fluorine, and filter to obtain the defluoridated liquid and filter residue containing calcium fluoride. The content of F in the liquid after defluoridation is 12mg / L, which can be directly cooled to crystallized Glauber's salt, and the calcium fluoride-containing filter residue is added to water at a solid-to-liquid ratio of 1:3g / mL, and added at a rate of 1.3 times the theoretical amount of calcium converted into calcium carbonate. Ammonium carbonate was ball milled at room temperature for 1.5 hours to make it completely transformed, and filtered to obtain a calcium carbonate filter cake and a solution containing ammonium fluoride. Then add sodium carbonate to the solution containing ammonium fluoride according...
Embodiment 3
[0041] Take the smelting fume leach solution with a pH of 4.2, stir and add calcium sulfate according to 2.5 times the theoretical amount of fluorine converted into calcium fluoride, filter to form a 3.5cm thick filter cake, and then circulate the filtrate to contact with the filter cake to maintain both The time of the first contact is 0.002h, and the room temperature is contacted for 2.5h to obtain the defluoridated liquid and the filter residue containing calcium fluoride. The F content in the liquid after defluoridation is 19mg / L, which can be directly used to separate and recover the valuable metals in it. The calcium fluoride-containing filter residue is added to water at a solid-to-liquid ratio of 1:2g / mL, and converted into calcium carbonate according to the calcium in it. Potassium carbonate was added to 1.3 times the theoretical amount, ball milled at room temperature for 1 hour to make it completely transformed, and filtered to obtain a calcium carbonate filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com