Application of a germanium-gallium nanowire as an electrode material for lithium-ion batteries
A lithium-ion battery, electrode material technology, applied in battery electrodes, nanotechnology, nanotechnology and other directions, can solve problems such as poor electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] In the present invention, the preparation method of the germanium gallium nanowire preferably includes the following steps:
[0036] In an environment where the water oxygen content is lower than 2ppm, the GaCl 3 、GeCl 4 Mixed with ionic liquid to obtain electrolyte;
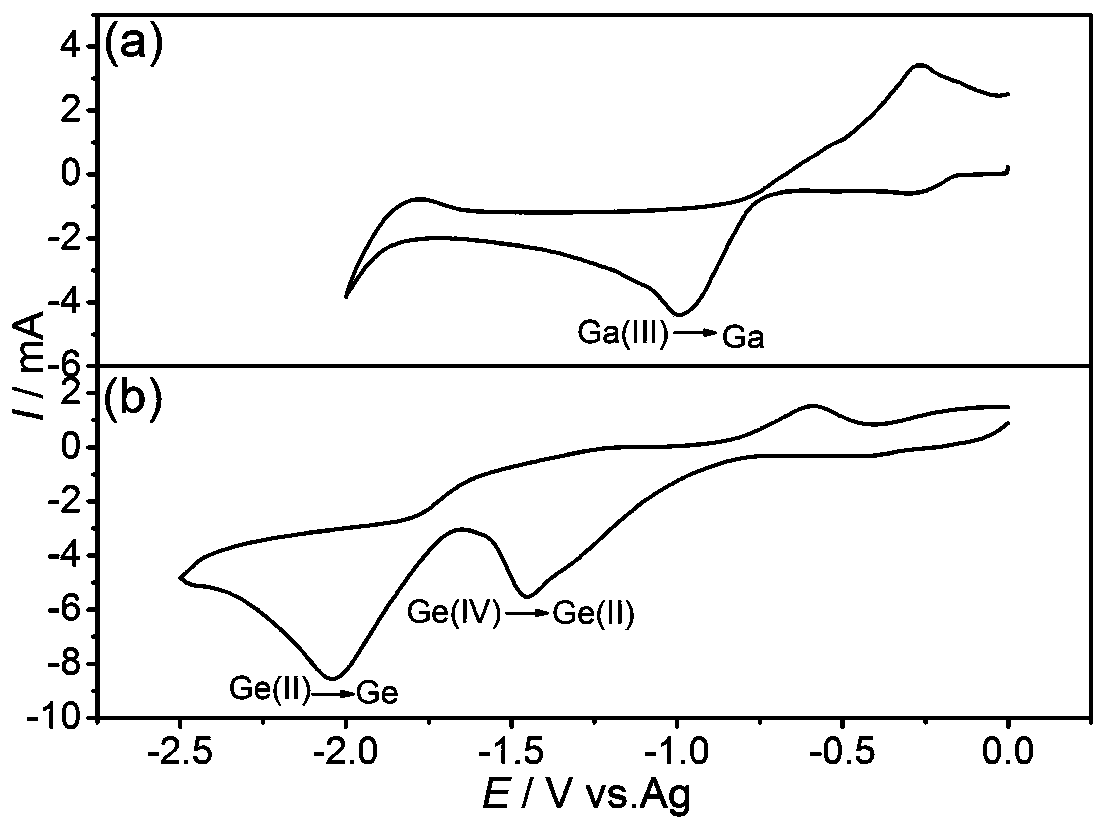
[0037] Using a three-electrode electrochemical system including a working electrode, a counter electrode and a reference electrode, the electrolyte is deposited at a constant voltage of gallium under the conditions of 55 to 65°C and -1.0 to -1.5V, and on the surface of the working electrode Gain gallium deposits; continue to electrodeposit germanium at a constant voltage under the conditions of 55-65°C and -1.8-2.2V, and obtain germanium-gallium nanowires on the surface of the working electrode.
[0038] In the present invention, the germanium-gallium nanowires are preferably prepared in an environment with a water and oxygen content lower than 2 ppm. In the present invention, the germanium gallium nan...
Embodiment 1
[0060] In a glove box filled with argon and with water and oxygen content lower than 2ppm, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIm]Tf 2 N) The ionic liquid was vacuum distilled at 100°C for 24h, and the resulting ionic liquid was mixed with GaCl 3 (99.999%) and GeCl 4 (99.9999%) mixed, stirred at 500rpm at room temperature for 12h to obtain 1mol / L GaCl 3 Electrolyte and 1mol / L GeCl 4 electrolyte;
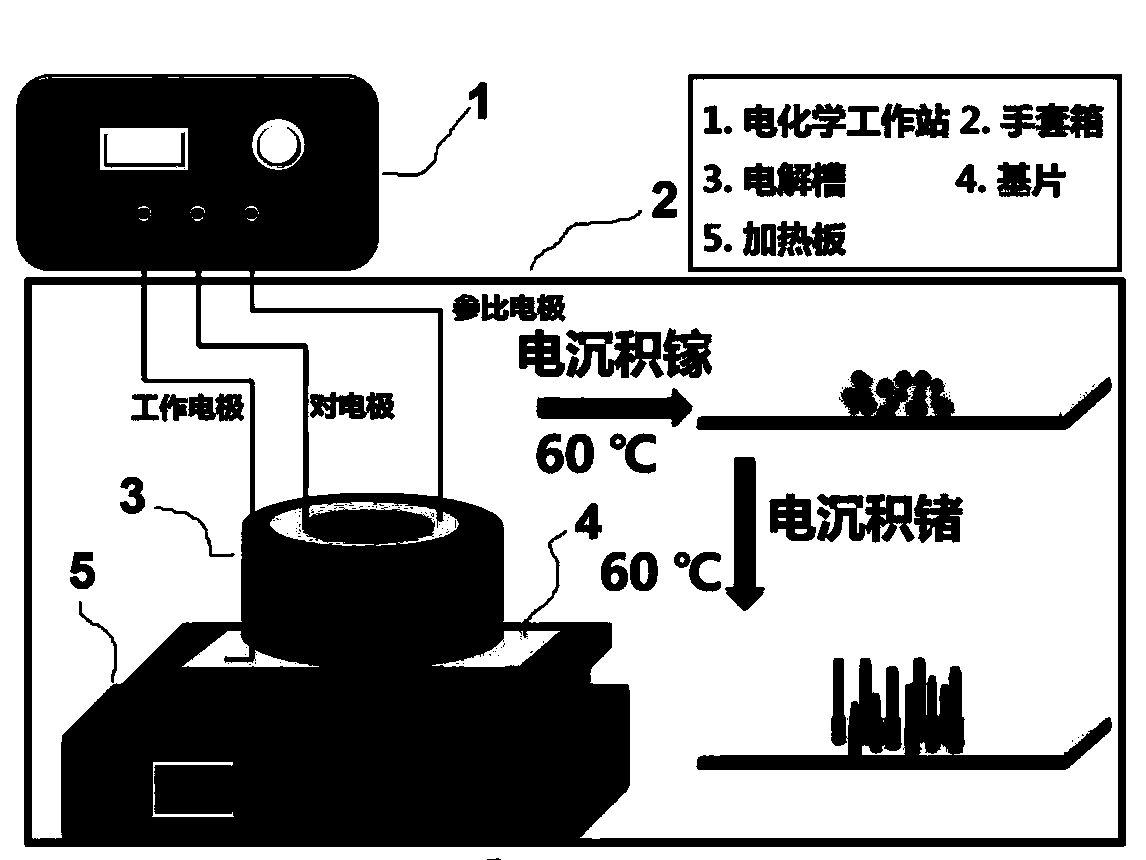
[0061] Clean the Cu substrate with acetone and isopropanol in turn, and then soak it in 10% hydrochloric acid for 1 min to remove the surface oxides. The Cu substrate is used as the working electrode, the silver wire is used as the reference electrode, and the platinum sheet is used as the counter electrode. Utilize a polytetrafluoroethylene electrolytic cell to limit the deposition area to 1.5cm 2 , using 2273 electrochemical workstation (Princeton Applied Research) to heat GaCl to 60 ° C 3 Electrolyte and GeCl 4 Electrolyte solution carries out c...
Embodiment 2
[0064] figure 2 It is a schematic diagram of the process of depositing germanium gallium nanowires on a Cu substrate, and the specific steps are as follows:
[0065] In a glove box filled with argon and with water and oxygen content lower than 2ppm, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIm]Tf 2 N) The ionic liquid was vacuum distilled at 100°C for 24h, and the resulting ionic liquid was mixed with GaCl 3 (99.999%) and GeCl 4 (99.9999%) mixed, stirred at 500rpm at room temperature for 12h to obtain GaCl 3 concentration and GeCl 4 Electrolyte with a concentration of 1mol / L;
[0066] Clean the Cu substrate with acetone and isopropanol in sequence, and then soak it in 10% hydrochloric acid for 1 min to remove the surface oxides. The Cu substrate is used as the working electrode, the silver wire is used as the reference electrode, and the platinum sheet is used as the counter electrode. Utilize a polytetrafluoroethylene electrolytic cell to limit t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com