Establishment of recombinant adeno-associated virus carrier production process
A virus vector and production process technology, applied in the field of establishment of recombinant adeno-associated virus vector production process, can solve the problems of many influencing factors, complicated operation, low immunogenicity, etc., and achieve stable and lasting expression, low immunogenicity, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of rAAV vector particles
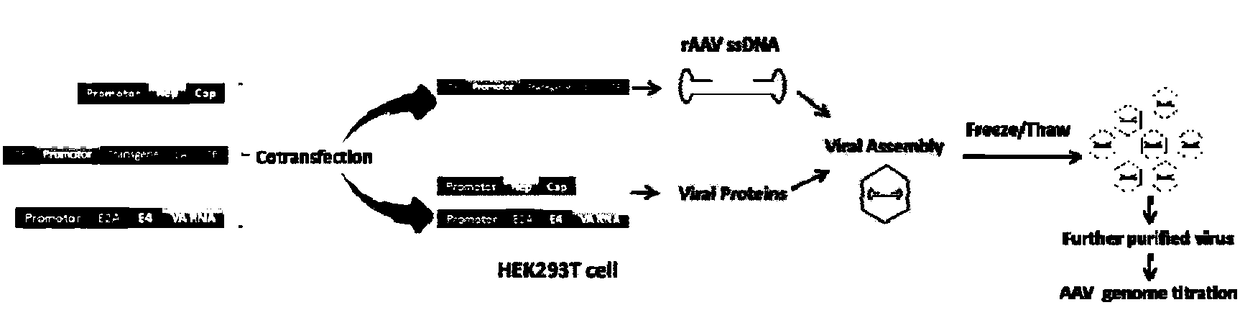
[0049] Such as image 3 Shown, the preparation method of a kind of rAAV vector particle in this implementation comprises the following steps:
[0050] (1) Culture of HEK293T cells:
[0051] Production of rAAV vector particles was performed in HEK293T. HEK293T cells were mixed at 1.4×10 per dish 6 The amount of cells was inoculated in a 10cm culture dish containing 15% (volume percent concentration) FBS DMEM medium, and placed at 37 ° C, 5% CO 2 cultured in an environment of saturated humidity. Transfection was performed 2 days after inoculation.
[0052] (2) Transfection of HEK293T cells:
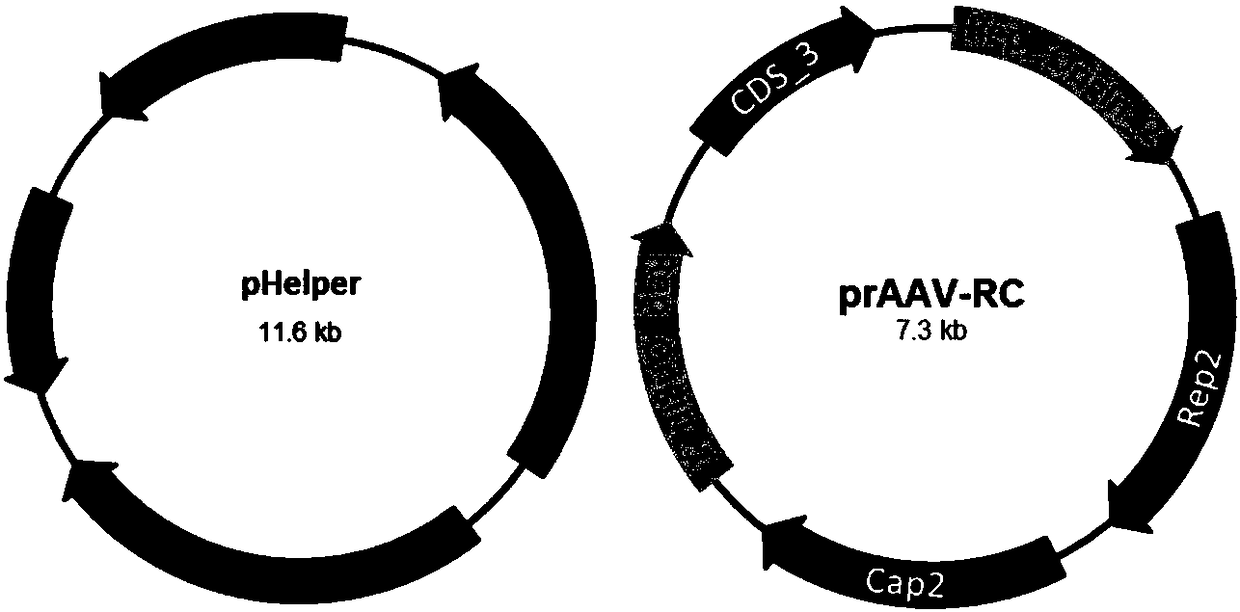
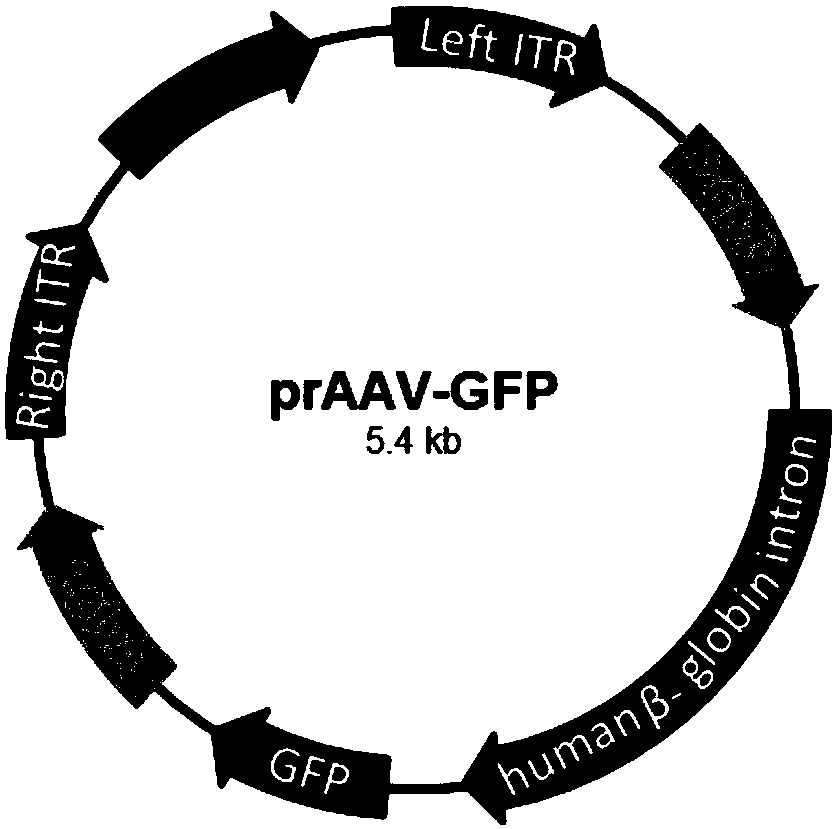
[0053] Before transfection, the cell culture medium was replaced with 5 ml of DMEM medium containing 5% (volume percent concentration) FBS. The two helper vector plasmids pHelper( Figure 1A ), prAAV-RC ( Figure 1A ) and a shuttle vector plasmid prAAV-GFP ( Figure 1B ) were co-transfected into HEK293T cells at a mass ratio of...
Embodiment 2
[0060] Example 2 Preparation of rAAV vector particles
[0061] Such as image 3 Shown, the preparation method of a kind of rAAV vector particle in this implementation comprises the following steps:
[0062] (5) Culture of HEK293T cells:
[0063] Production of rAAV vector particles was performed in HEK293T. HEK293T cells were mixed at 1.4×10 per dish 6 The amount of cells was inoculated in a 10 cm culture dish containing 8% (volume percent concentration) FBS DMEM medium, and placed at 37 ° C, 5% CO 2 cultured in an environment of saturated humidity. Transfection was performed 2 days after inoculation.
[0064] (6) Transfection of HEK293T cells:
[0065] Before transfection, the cell culture medium was replaced with 5 ml of DMEM medium containing 2% (volume percent concentration) FBS. The two helper vector plasmids pHelper( Figure 1A ), prAAV-RC ( Figure 1A ) and a shuttle vector plasmid prAAV-GFP ( Figure 1B ) were co-transfected into HEK293T cells at a mass ratio of...
Embodiment 3
[0072] Example 3 Preparation of rAAV vector particles
[0073] Such as image 3 Shown, the preparation method of a kind of rAAV vector particle in this implementation comprises the following steps:
[0074] (9) Culture of HEK293T cells:
[0075] Production of rAAV vector particles was performed in HEK293T. HEK293T cells were mixed at 1.4×10 per dish 6 The amount of cells was inoculated in a 10cm culture dish containing 20% (volume percent concentration) FBS DMEM medium, and placed at 37 ° C, 5% CO 2 cultured in an environment of saturated humidity. Transfection was performed 2 days after inoculation.
[0076] (10) Transfection of HEK293T cells:
[0077] Before transfection, the cell culture medium was replaced with 5 ml of DMEM medium containing 10% (volume percent concentration) FBS. The two helper vector plasmids pHelper( Figure 1A ), prAAV-RC ( Figure 1A ) and a shuttle vector plasmid prAAV-GFP ( Figure 1B ) were co-transfected into HEK293T cells at a mass rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com