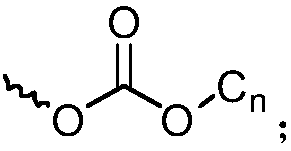
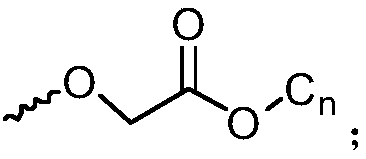
Octaphenyl substituted polyhedral oligomeric silsesquioxane derivative molecular glass and application thereof
A technology of silsesquioxane and molecular glass, which is applied in the field of materials, can solve the problems of poor product repeatability, insufficient purity, and low modification yield, and achieve the effects of high yield, simple synthesis process, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] A preparation method of octaphenyl substituted clathrate silsesquioxane derivative molecular glass, comprising the following steps:
[0079] 1) Preparation of octa-(7,8-dimethoxybiphenyl)silsesquioxane, the reaction formula of the synthetic route is as follows:
[0080]
[0081] Under the protection of high-purity nitrogen, add octa(p-iodophenyl substituted) silsesquioxane (20.4g, 10mmol, 1.0eq) in a 500ml schleck reaction flask, 200ml of redistilled tetrahydrofuran, stir and dissolve, and pour into the reaction flask Add 3,4-dimethoxyphenylboronic acid (18.2g, 100mmol, 10.0eq) and 2M Na 2 CO 3 Aqueous solution 80ml, finally add catalyst Pd (PPh 3 ) 4 (577mg, 0.5mmol, 0.05eq), the reaction solution was heated to 50-70°C for 12h, cooled to room temperature, the reaction solution was extracted with dichloromethane / water, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent , the residue was...
Embodiment 2
[0091] A preparation method of octaphenyl substituted clathrate silsesquioxane derivative molecular glass, comprising the following steps:
[0092] 1) Preparation of octa-(7,8,9-trimethoxybiphenyl)silsesquioxane, the reaction formula of the synthetic route is as follows:
[0093]
[0094] Under the protection of high-purity nitrogen, add octa(p-iodophenyl substituted) silsesquioxane (20.4g, 10mmol, 1.0eq) in a 500ml schleck reaction flask, 200ml of redistilled tetrahydrofuran, stir and dissolve, and pour into the reaction flask Add 3,4,5-trimethoxyphenylpinacolborane (29.4g, 100mmol, 10.0eq) and 2M Na 2 CO 3 Aqueous solution 80ml, finally add catalyst Pd (PPh 3 ) 4 (577mg, 0.5mmol, 0.05eq), the reaction solution was heated to 50-70°C for 12h, cooled to room temperature, the reaction solution was extracted with dichloromethane / water, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent , the resi...
Embodiment 3
[0104] Step 1) and 2) are the same as in Example 1.
[0105] 3) Octa-(7,8-diadamantyl biphenyl)silsesquioxane, the reaction formula of the synthetic route is as follows:
[0106]
[0107] AD in the reaction formula represents Substituents.
[0108] Add octa-(7,8-dihydroxybiphenyl)silsesquioxane (4.0, 2.1mmol, 1.0eq), tetrabutylammonium bromide (812mg, 2.5mmol, 1.2eq) into a 100mL three-necked flask, K 2 CO 3 (4.6g, 33.6mmol, 16.0eq) and N-methylpyrrolidone (NMP, 40ml), stir evenly at room temperature, slowly add adamantyl chloroacetate (8.15g, 33.6mmol, 16.0eq) dropwise to the reaction solution NMP (20ml) solution, the temperature of the reaction system was raised to 60°C for 48h. After the reaction is complete, cool to room temperature, the reaction solution is extracted with ethyl acetate / water, the organic phase is washed once with 3wt% oxalic acid solution and water respectively, the organic layers are combined, dried over anhydrous magnesium sulfate, the solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com