Fusion protein for restoring function of failing immune cell and application thereof
A technology of immune cells and fusion proteins, applied in the field of fusion proteins, can solve problems such as toxic side effects and off-target effects, achieve a wide range of applications, enhance the effect of inhibiting tumor growth, and have good clinical prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
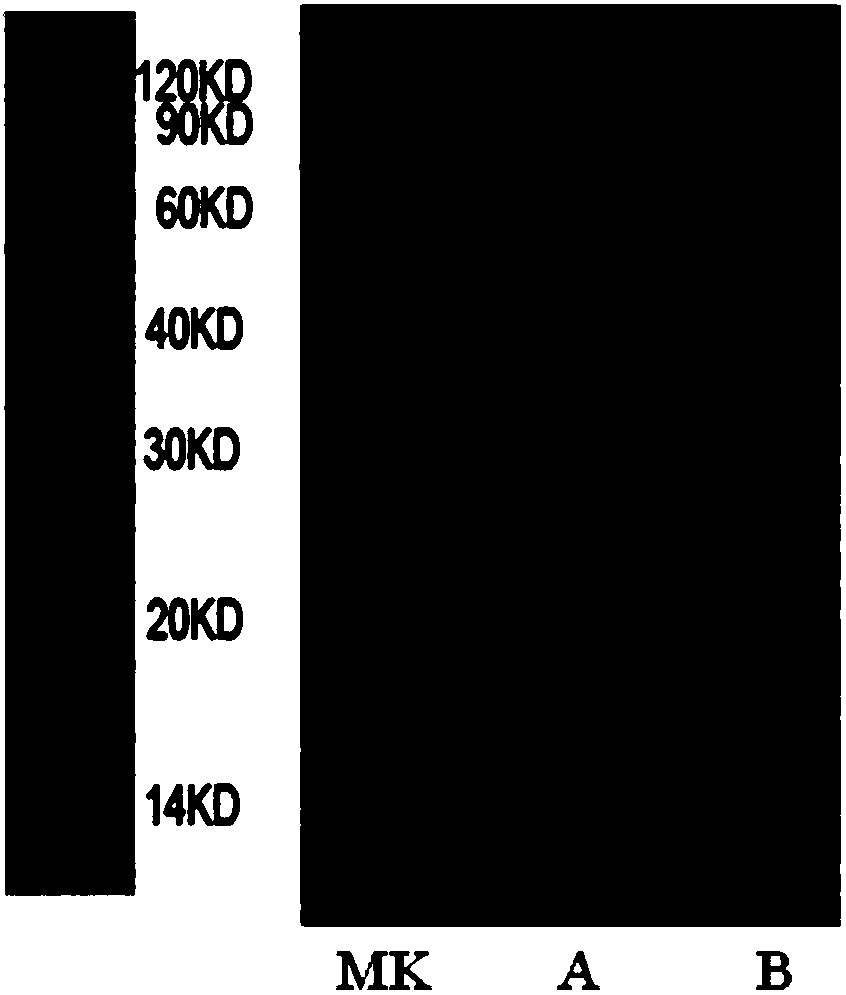
[0045] This example is the gene construction and production purification of the recombinant fusion protein.
[0046] According to the C-terminal amino acid sequence of human PD-1 single-chain antibody (SEQ ID NO: 2) and the N-terminal amino acid sequence of interleukin 2 (SEQ ID NO: 3), through gene synthesis, enzyme digestion and further cloning, non-functional artificially constructed amino acids ( SEQ ID NO: 4) The two parts are connected to form the artificially constructed gene sequence of the fusion protein (SEQ ID NO: 5), and then transferred into the eukaryotic cell expression vector pcDNA3.1(-). Finally, the expression vector of the fusion protein was transfected into Chinese hamster ovary cells (CHO). Transfected cells were placed at 37°C, 5% CO 2 After culturing in an incubator, the supernatant was taken after 72 hours, and further purified by Protein A affinity chromatography. The final purified protein was a bifunctional recombinant fusion artificial protein (αPD...
Embodiment 2
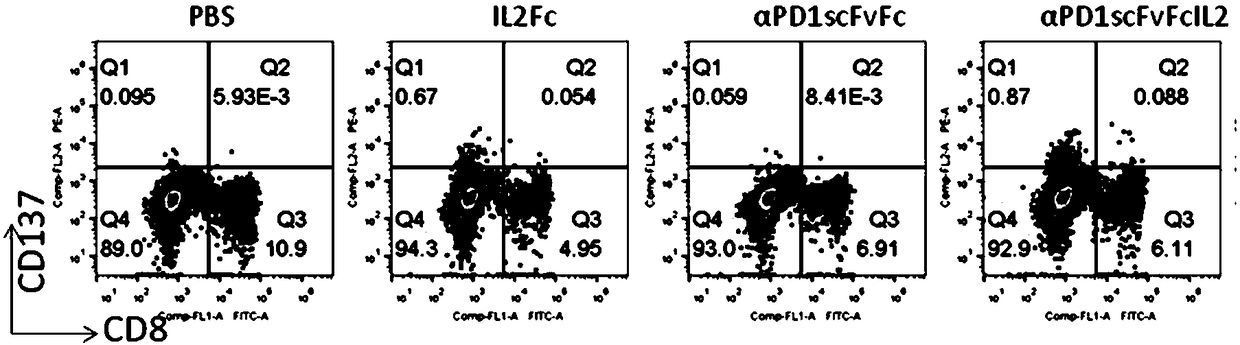
[0048] This example is the activity and function determination of the bifunctional recombinant fusion protein on human PBMC cells cultured in vitro.
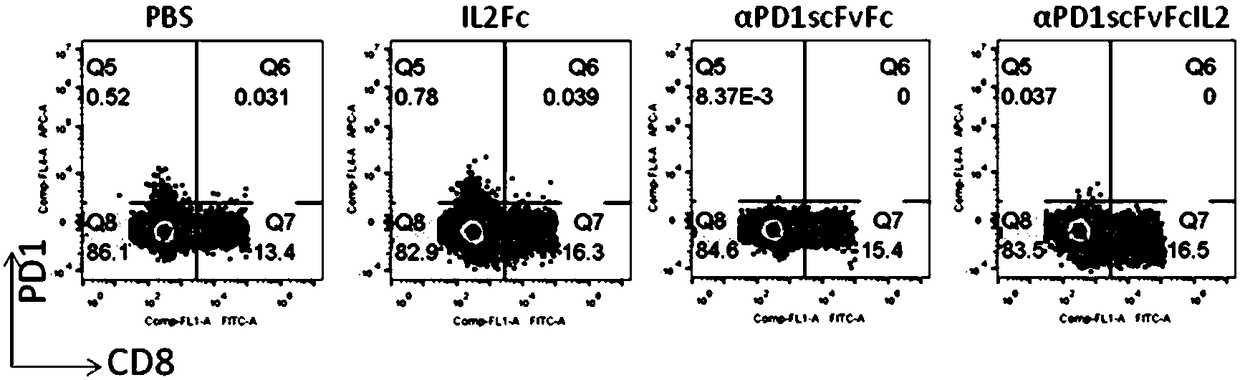
[0049] Human peripheral blood was separated and purified by lymphocyte density gradient centrifugation (Ficoll), and diluted with X-Vivo15 medium in a 24-well plate to a cell density of 5×10 6 / ml, the test protein was added to a final concentration of 200ng / ml. Then placed at 37°C, 5% CO 2 Cultured in the incubator for 72 hours, the cells were collected, stained with flow cytometry antibodies, and washed with flow cytometry for phenotypic determination and data analysis. figure 2 showed that compared with the control group without protein addition, PD-1 antibody (αPD1scFvFc) could not induce the expression of CD137 (0.06% vs 0.09%), while fusion protein (αPD1scFvFcIL2) treatment could significantly increase the expression of CD137 in CD8- and CD8+ cells Expression (0.96%). At the same time, if image 3 It showed that the P...
Embodiment 3
[0051] This example is the determination of the activity and function of the bifunctional recombinant fusion protein on human ascites immune cells.
[0052] The ascites of human lung cancer patients was taken, and the test protein was added to a 24-well plate to a final concentration of 200ng / ml. Then placed at 37°C, 5% CO 2 After being cultured in the incubator for 72 hours, the cells were collected and stained with CD8 and PD-1 flow cytometry antibodies, and then perforin was used to break the cell membrane for internal staining of TNFα and IFNγ flow cytometry antibodies in CD8+ cells, washed and stained with flow cytometry antibodies instrument for phenotyping and data analysis. Such as Figure 4 showed that fusion protein (αPD1scFvFcIL2) treatment significantly increased the expression of TNFα in ascites PBMCs (13.0% vs 5.29%) compared with unfused PD-1 antibody (αPD1scFvFc).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com