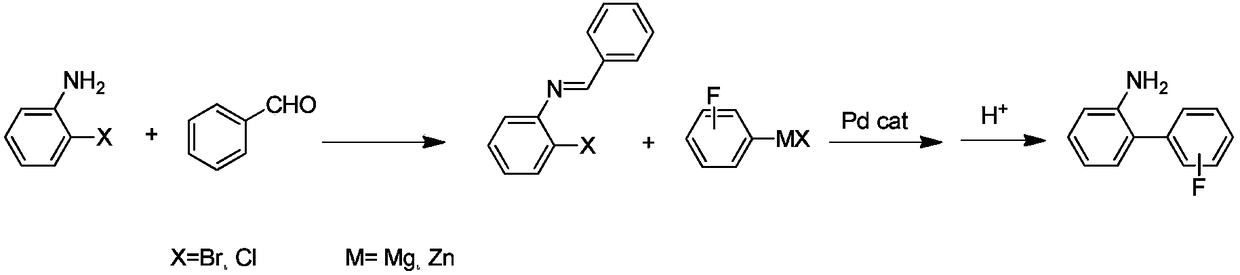
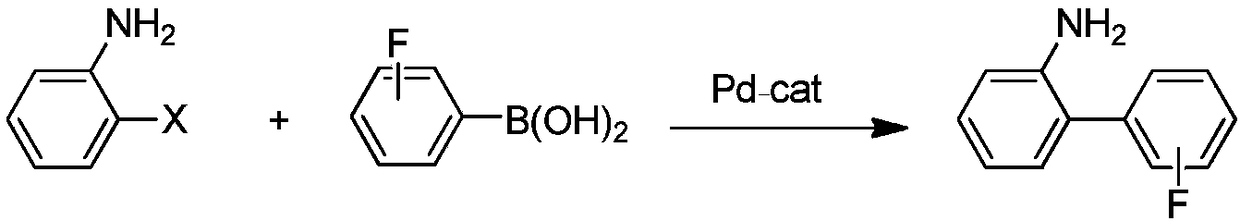Preparation method of fluorine-containing benzidine
A technology of fluorobenzidine and benzidine, which is applied in the field of preparation of fluorine-containing benzidine, and can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] In the preparation method of fluorine-containing benzidine provided by the present invention, it may comprise: preparing the compound of formula I after coupling the compound of formula VI with the compound of formula IV, acidic hydrolysis, and the reaction equation is as follows:
[0095]
[0096] Wherein, Hal is selected from Br, Cl;
[0097] M is selected from Mg, Zn;
[0098] X is selected from Cl, Br, I, preferably Cl;
[0099] R 7 Selected from H, F, C1-C4 alkyl, C1-C4 alkoxy;
[0100] R 8 Selected from H, phenyl, C1-C4 alkyl, preferably H.
[0101] In the preparation method of fluorine-containing benzidine provided by the present invention, coupling reaction is carried out under the condition that catalyst exists usually, and the catalyzer used in coupling reaction can be nickel series catalyst usually, for example, can be nickel salt (for example, Can be a divalent nickel salt, more specifically can be for example NiCl 2 , nickel bromide, Ni(OAc) 2 etc...
Embodiment 1
[0139] Preparation of N-benzylidene-2-chloroaniline:
[0140] Under nitrogen protection, install a water separator in a 2L four-necked bottle, add 127.5g of 2-chloroaniline, 116g of benzaldehyde, 12.7g of p-toluenesulfonic acid and 1L of cyclohexane, reflux for 6 hours of water separation reaction, and there is no The reaction can be stopped when obvious water droplets are produced. After cooling to room temperature, add 100mL of 10% sodium hydroxide aqueous solution to wash, the organic phase is desolvated under reduced pressure, and the resulting residue is distilled under reduced pressure at 10mmHg to obtain 196.6g of the target product, N-benzylidene-2-chloroaniline, with a gas chromatography content of 99.0% , yield 91.2%.
Embodiment 2
[0142] Preparation of N-benzylidene-2-chloro-4-methylaniline:
[0143] Under the protection of nitrogen, install a water separator in a 2L four-neck bottle, add 141.6g of 2-chloro-4-methylaniline, 116g of benzaldehyde, 7.0g of p-toluenesulfonic acid and 1.2L of hexane, reflux for 6h, and separate The reaction can be stopped when there is no obvious water drop in the water container. After cooling to room temperature, 100 mL of 10% aqueous sodium hydroxide solution was added for washing, the organic phase was desolvated under reduced pressure, and the resulting residue was distilled under reduced pressure at 10 mmHg to obtain 206.7 g of the target product N-benzylidene-2-chloro-4-methylaniline. The gas chromatography content is 98.9%, and the yield is 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com