Loading type composite metal-acid bifunctional catalyst
A bifunctional catalyst, composite metal technology, applied in molecular sieve catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve problems such as catalyst deactivation, achieve simple preparation process, simple reaction process, excellent activity and orientation selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Catalyst preparation
[0022] Preparation of one or more modified carriers among lanthanum, cerium, praseodymium and neodymium: Weigh 1g of activated carbon, alumina, titanium dioxide, Y-type molecular sieve, ZSM-5, β-molecular sieve and other carriers, and add them to NH4Cl Mix and stir in the solution evenly, then add LaCl3, CeCl3, PrCl3, NdCl3 aqueous solutions with 5wt% equivalents of rare earth elements respectively, and keep the obtained mixed solution in a water bath at 80°C for 1 hour, filter, wash, and then calcined at 600°C After 2 hours, the preparation of a carrier modified by one or more of lanthanum, cerium, praseodymium and neodymium (active component B) was obtained.
[0023] Preparation of supported metal-acid bifunctional catalyst (active component A+B):
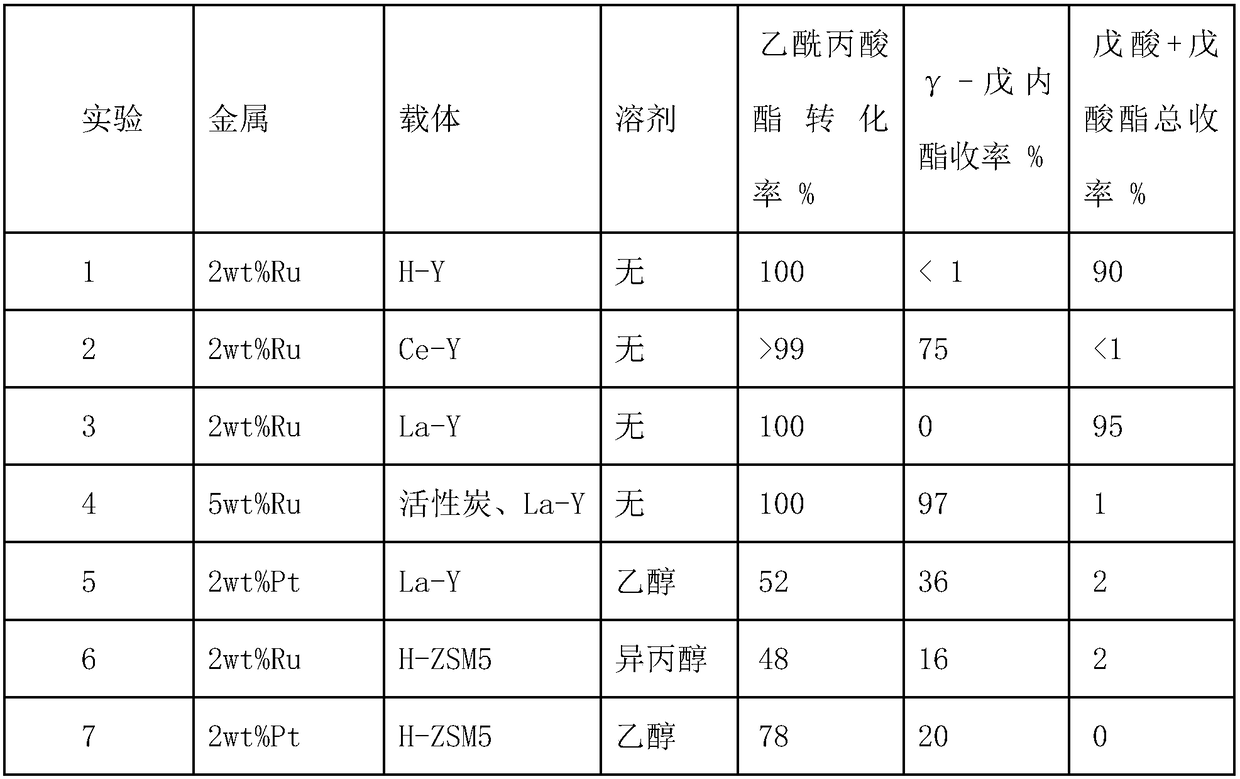
[0024] Experiment 1: Preparation of metal catalyst 2wt% Ru / H-Y: Weigh 0.1250 g of hexaammine ruthenium chloride in a clean 50 ml round bottom flask, add 25 ml of ultrapure water, place the round bot...
Embodiment 2
[0031] Controlled experiment
[0032] Experiment 4: The catalyst similar to Example 1 is used for the preparation of composite catalyst Ru / C+La-Y: take 5wt% Ru / C 0.1g respectively, La-Y molecular sieve 0.4g, obtain composite catalyst through mechanical mixing Ru / C+La-Y. Under the same experimental conditions, although the non-supported catalyst Ru / C+La-Y can realize the complete conversion of levulinic acid ester, the selectivity of valeric acid (ester) is not high, only 2%.
[0033] Experiment 5-7: The preparation method is the same as in Example 1, and 2wt% Pt / La-Y, 2wt% Ru / H-ZSM5 and 2wt% Pt / H-ZSM5 are prepared. Under the condition of adding solvent, the selectivity of valeric acid (ester) is not high.
[0034] Catalyst activity test: add 5g of ethyl levulinate or 2g of ethyl levulinate with 18g of solvent, 0.5g of catalyst into a 50ml reaction kettle, replace the gas with nitrogen for three times, then replace with hydrogen for three times, and then fill with hydrogen t...
Embodiment 3
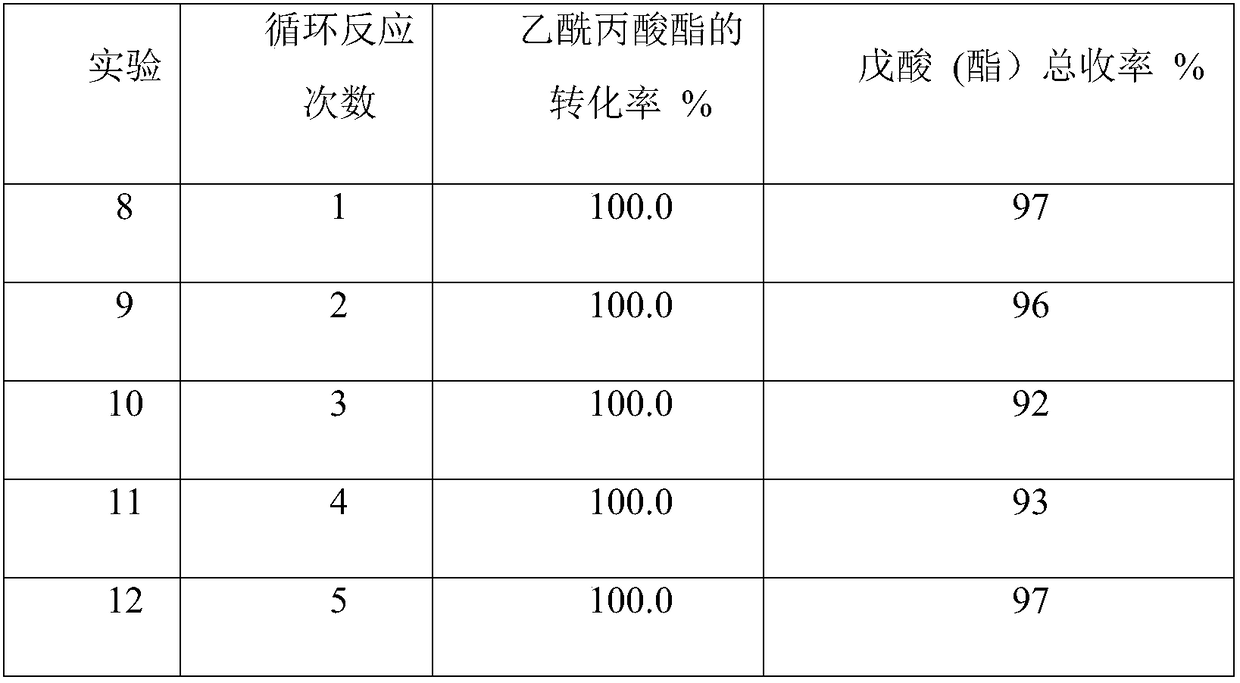
[0036] The conversion effect of the 2wt%Ru / La-Y catalyst for the selective hydrogenation of ethyl levulinate in five cycles of experiments is shown in Table 2:
[0037] Catalyst cycle test: Add 5g of ethyl levulinate and 0.5g of catalyst into a 50ml reactor, pass through nitrogen to replace the gas three times, then pass through hydrogen to replace three times, then fill with hydrogen to 4MPa, heat up to 220°C and stir for 10h. After the reaction, the catalyst was filtered, recovered, washed three times with ethanol, air-dried, and then put into the next cycle reaction.
[0038] Table 2 1wt%Ru / La-Y Catalyst Cycle Experiment Results
[0039]
[0040] The 2wt% Ru / La-Y catalyst still achieves complete conversion to ethyl levulinate after five cycles of experiments, and the total yield of valeric acid and valeric acid esters remains above 90%. This catalyst has excellent stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com