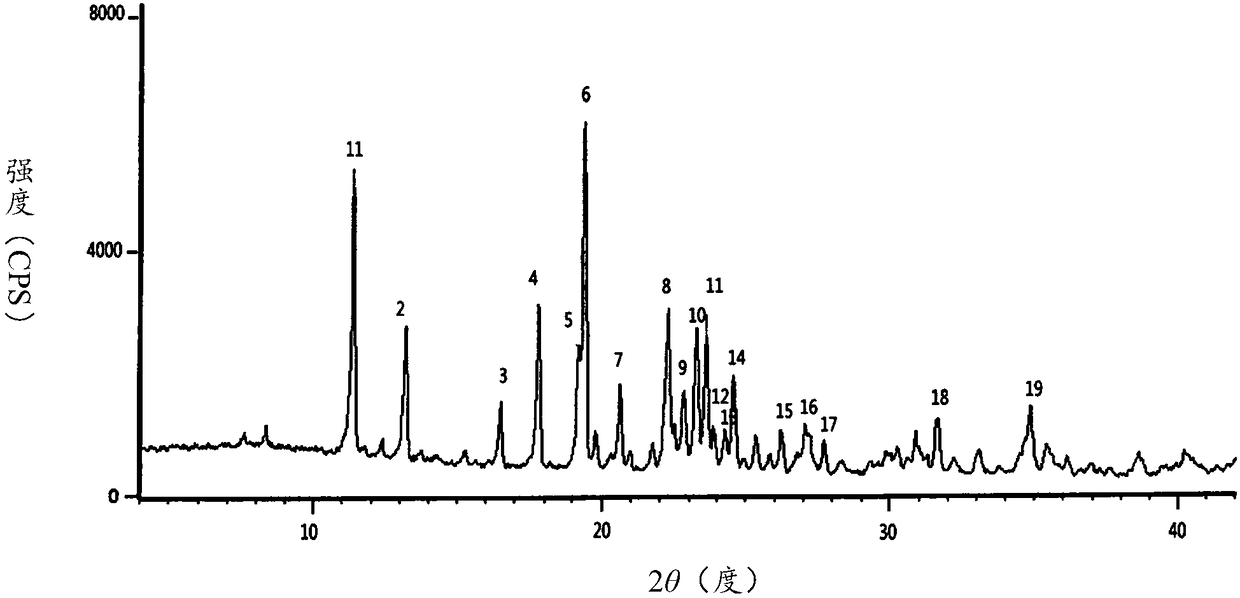
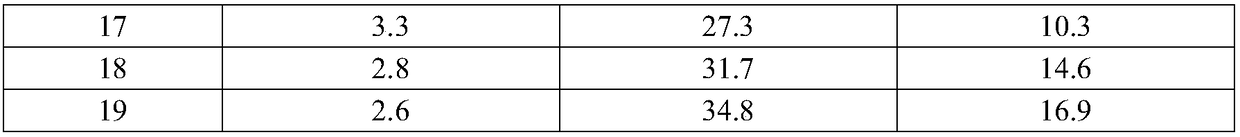
Ceftezole sodium compound and preparation thereof
A technology of ceftezole sodium and its compound, which is applied in the field of ceftezole sodium compound and its preparation, and can solve the problems of β-lactam ring opening, dark color, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of ceftezole sodium compound
[0025] (1) Dissolve 2.56kg tetrazoleacetic acid and 2.43kg 2-mercapto-1,3,4-thiadiazole in tetrahydrofuran, slowly add 5.04kg N,N'-dicyclohexylcarbodiimide, ice React in the bath for 2 hours, remove 1,3-dicycloethylurea by filtration, concentrate the filtrate under reduced pressure, add 25L of chloroform, stir, filter and dry to obtain 4.1Kg of active ester as a yellow powder;
[0026] (2) Add 4.44Kg 7-ACA to 60L of chloroform and triethylamine with a volume ratio of 2:1, add the above-mentioned active ester to react for 5h under ice-bath conditions, add 400L of water, separate the water layer, and use 2mol / L of glacial acetic acid solution to adjust the pH to 2.0, stir in an ice bath for 1 h, filter, wash with purified water, and dry in vacuo to obtain 6.23 Kg of 7-(1H-tetrazolium acetamido) cephalosporanic acid;
[0027] (3) Add 50L acetic acid to 7-(1H-tetrazolium acetylamino) cephalosporanic acid and 2.0...
Embodiment 2
[0036] Embodiment 2: the preparation of ceftezole sodium compound
[0037] (1) Dissolve 30.33g of tetrazoleacetic acid and 31.11g of 2-mercapto-1,3,4-thiadiazole in tetrahydrofuran, slowly add 61.77g of N,N'-dicyclohexylcarbodiimide, ice React in the bath for 2 hours, remove 1,3-dicycloethylurea by filtration, concentrate the filtrate under reduced pressure, add 700ml of tetrahydrofuran, stir, filter and dry to obtain 59.24g of active ester as a yellow powder;
[0038](2) Add 58.34g of 7-ACA into 1400ml of chloroform and triethylamine with a volume ratio of 15:2, add the above-mentioned active ester to react for 4h under ice-bath conditions, add 600ml of water, separate the water layer, and use 1mol / L of hydrochloric acid solution to adjust the pH to 2.5, stir in an ice bath for 1 h, filter, wash with purified water, and dry in vacuo to obtain 94.79 g of 7-(1H-tetrazolium acetamido) cephalosporanic acid;
[0039] (3) Add 1500ml of acetic acid to 7-(1H-tetrazolium acetylamino...
Embodiment 3
[0042] Embodiment 3: the preparation of ceftezole sodium compound
[0043] (1) Dissolve 32.06g of tetrazoleacetic acid and 30.79g of 2-mercapto-1,3,4-thiadiazole in tetrahydrofuran, slowly add 60.42g of N,N'-dicyclohexylcarbodiimide, ice React in the bath for 2 hours, remove 1,3-dicycloethylurea by filtration, concentrate the filtrate under reduced pressure, add 700ml of absolute ethanol, stir, filter and dry to obtain 60.33g of active ester as a yellow powder;
[0044] (2) Add 60.12g of 7-ACA to 1500ml of chloroform and triethylamine with a volume ratio of 5:1, add the above-mentioned active ester to react for 4h under ice-bath conditions, add 600ml of water, separate the water layer, and use 1mol / L of nitric acid solution to adjust the pH to 3.0, stir in an ice bath for 1 h, filter, wash with purified water, and dry in vacuo to obtain 94.23 g of 7-(1H-tetrazolium acetamido) cephalosporanic acid;
[0045] (3) Add 1500ml of acetic acid to 7-(1H-tetrazolium acetylamino) cepha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com