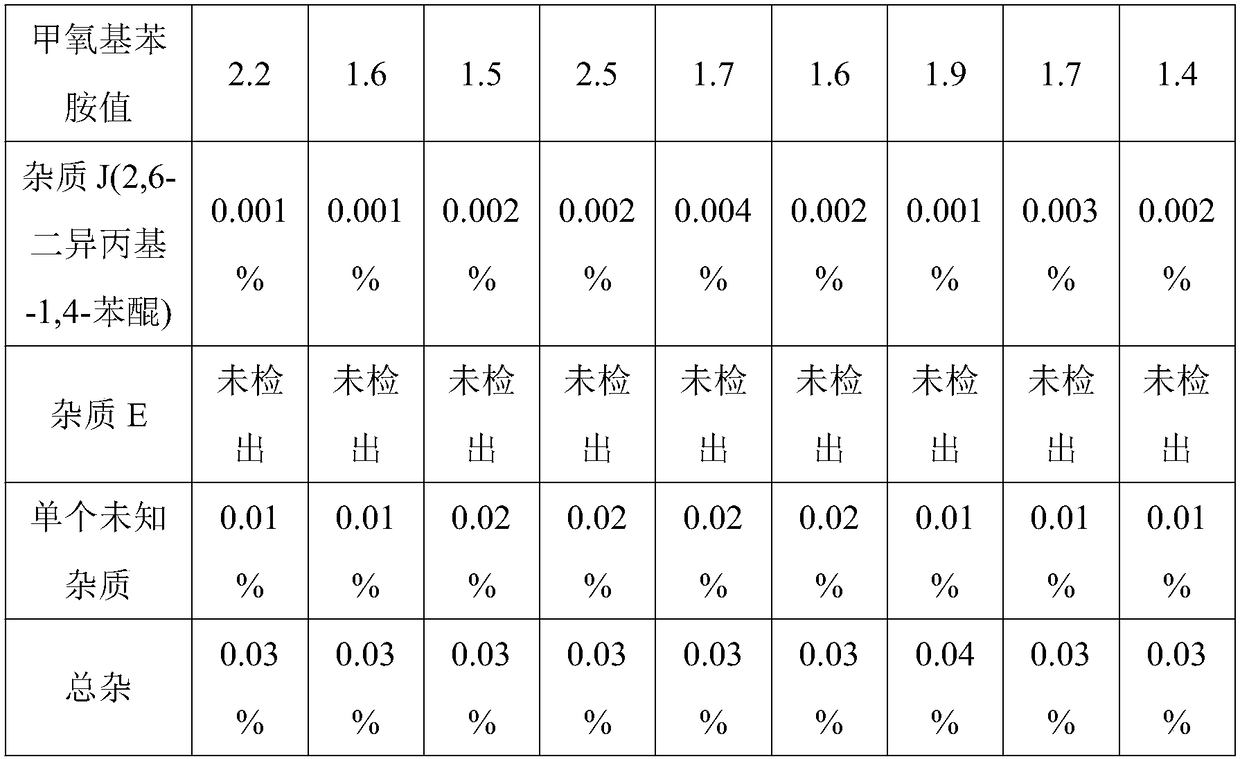
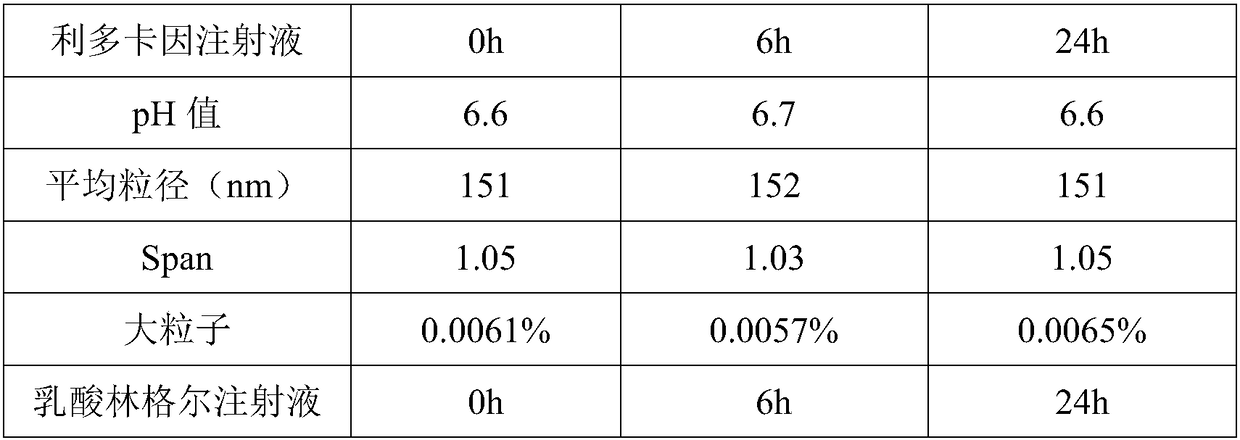
Propofol injection and preparation method thereof
A technology of propofol and injection, which is applied in the field of medicine, can solve problems such as interference with metabolism, and achieve the effects of improving safety, improving encapsulation efficiency, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of structural triglycerides:
[0040] The quality is 879.4g triglycerin trioleate and the quality are 554.9g capric acid triglyceride to mix and then add in the airtight vacuum reaction container, the mixture of the obtained glycerol trioleate and capric triglyceride is vacuum Heat the reaction vessel to 80°C, then add 6.0 g of sodium methoxide to carry out transesterification reaction, stop heating after 50 min, and finally add 140 mL of citric acid aqueous solution with a concentration of 1 mol / L to terminate the reaction to obtain a crude triglyceride. Structural triglycerides are washed with water, centrifuged, and vacuum-dried to obtain the product.
Embodiment 2
[0041] Embodiment 2, a kind of propofol injection
[0042] The propofol injection consists of the following components:
[0043] Propofol 7g, structural triglyceride 70g, egg yolk lecithin 10g, isotonic regulator 20g, water for injection to 1000ml, adding disodium hydrogen phosphate to adjust the pH value to 7.9;
[0044] The preparation method is:
[0045] S1. In a nitrogen environment, weigh structural triglycerides, egg yolk lecithin and propofol, place them in a container, heat to 50°C, and shear until the phospholipids dissolve as oil under high-speed shear at a speed of 8000rpm / min. Mutually;
[0046] S2. In a nitrogen environment, add the isotonic regulator to water for injection with no less than 80% of the prescription amount, mix well, and then filter to form an aqueous phase;
[0047] S3. Under a nitrogen environment, adjust the oil phase and the water phase through a peristaltic pump, so that the volume ratio of the oil phase and the water phase is 1:3, and adju...
Embodiment 3
[0049] Embodiment 3, a kind of propofol injection
[0050] The propofol injection consists of the following components:
[0051] Propofol 10g, structural triglyceride 100g, egg yolk lecithin 12g, isotonic regulator 22.5g, water for injection to 1000ml, adding disodium hydrogen phosphate to adjust the pH value to 7.0;
[0052] The preparation method is similar to Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com