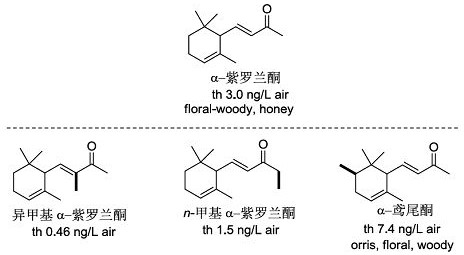
A kind of spice based on ɑ-ionone derivative and preparation method thereof
A technology of ionone and derivatives, which is applied in the field of novel fragrances based on ɑ-ionone derivatives and their preparation, and achieves the effects of environmental friendliness, high regio- and stereoselectivity, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
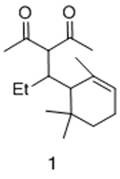
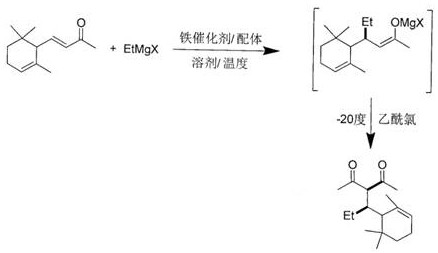
[0026] Add ferric acetylacetonate (0.02 mmol) and ligand hexamethylphosphoric triamide (0.05 mmol) into the reaction tube, and add solvent toluene (5 ml) and α-ionone ( 0.5 mmol), placed at 0°C, and under stirring, a solution of ethylmagnesium bromide (1 mmol) in ether was added dropwise to the reaction tube, and the reaction was continued at 0°C. After 3 hours, gas chromatography detected that the reaction was complete, cooled the reaction tube to -20 degrees, slowly added acetyl chloride solution (1 mmol), and continued to maintain the temperature for 1 hour after the dropwise addition, and then added saturated ammonium chloride solution dropwise The reaction was quenched, returned to room temperature and water was added. Extract with ether, wash the organic phase with 1% hydrochloric acid, saturated sodium bicarbonate and saturated brine successively, then dry with anhydrous sodium sulfate, filter, concentrate, flash column chromatography to obtain 3-(1-(2, 6,6-trimethyl-2...
Embodiment 2
[0028] Add ferric chloride (0.02 mmol) and ligand triphenylphosphine (0.2 mmol) into the reaction tube, and add solvent diethyl ether (5 ml) and α-ionone (1 mmol ), placed at a temperature of -10 degrees, and added dropwise a tetrahydrofuran solution of ethylmagnesium chloride (1.5 mmol) to the reaction tube under stirring, and continued the reaction at -10 degrees. After 6 hours, gas chromatography detected that the reaction was complete, cooled the reaction tube to -20 degrees, slowly added acetyl chloride solution (1.5 mmol), and continued to maintain the temperature for 1 hour after the dropwise addition, and then added saturated ammonium chloride solution dropwise The reaction was quenched, returned to room temperature and water was added. Extract with ether, wash the organic phase with 1% hydrochloric acid, saturated sodium bicarbonate and saturated brine successively, then dry with anhydrous sodium sulfate, filter, concentrate, flash column chromatography to obtain 3-(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com