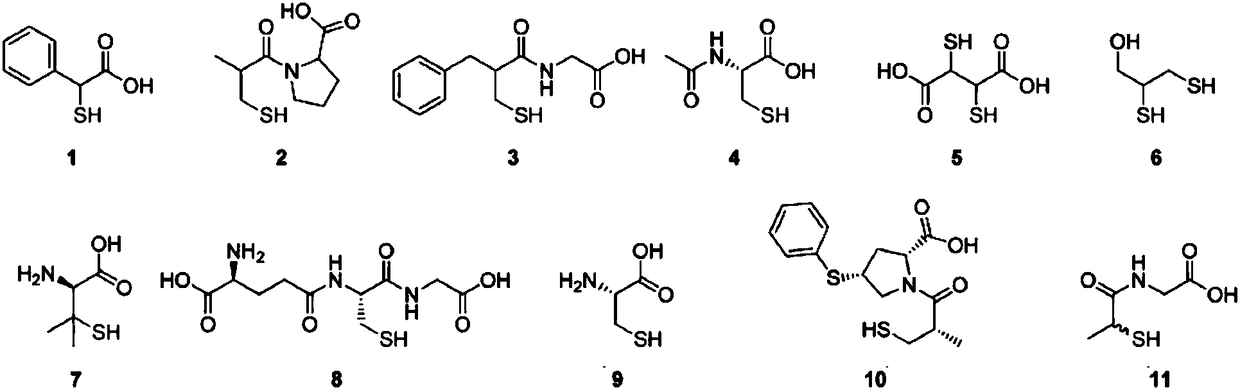
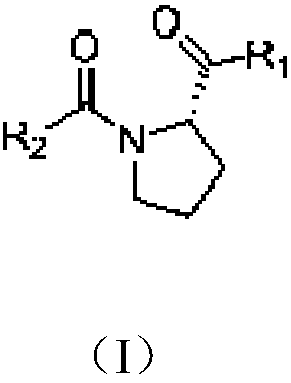
Use of proline derivatives in preparation of beta-lactamase inhibitors
A technology of lactamase and derivatives, which is applied in the direction of medical preparations containing active ingredients, antibacterial drugs, organic active ingredients, etc., can solve the unpleasant smell of drugs, and is not suitable for inhibiting super-resistant bacteria metal β-lactam, Drug toxicity and side effects and other problems, to achieve the effect of treating drug-resistant bacterial infections, good drug application prospects, and alleviating drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
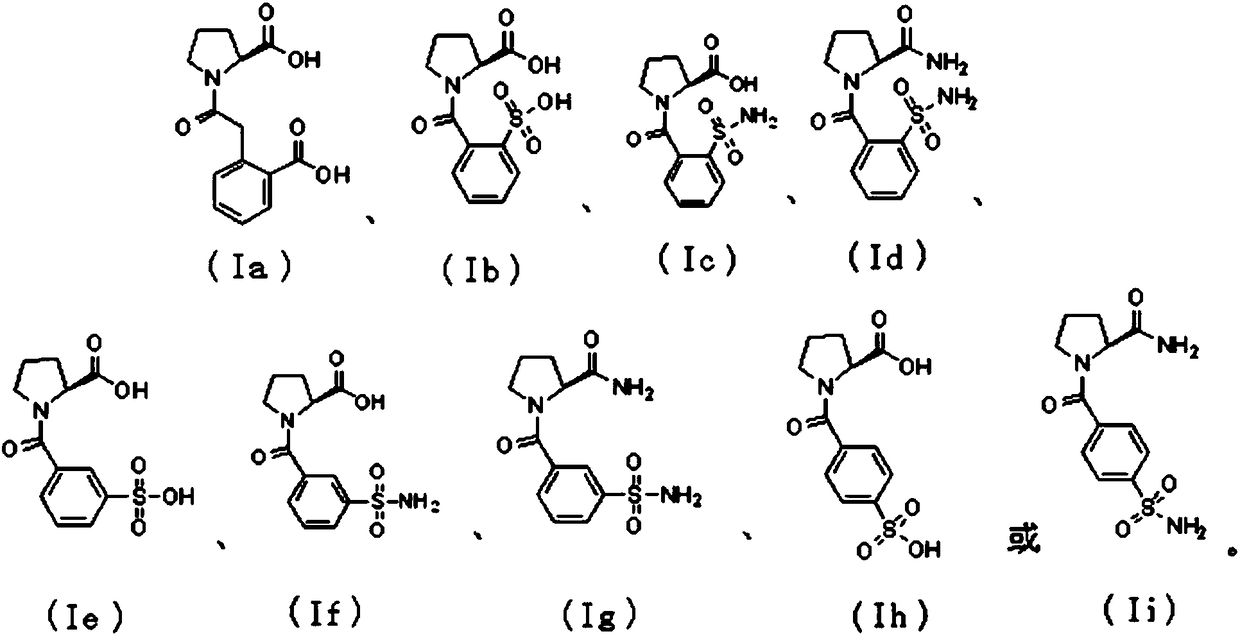
[0030] Embodiment 1: the synthesis of compound Ia:
[0031]
[0032](1) Dissolve o-carboxyphenylacetic acid (360mg, 2mmol) in dry dimethylformamide solution (10ml) at room temperature, and add ethyl[3-(dimethylamino)propyl]carbodiol successively Imine (384mg, 2mmol) and 1-hydroxybenzotriazole (270mg, 2mmol), stirred for 15 minutes, then added L-proline benzyl ester hydrochloride (532mg, 2.2mmol) and 4-dimethylaminopyridine (805mg, 6.6mmol), the reaction solution was reacted at room temperature for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, the reactant was diluted with dichloromethane, washed with saturated sodium bicarbonate, 3M hydrochloric acid solution and saturated sodium chloride solution respectively, and the organic phase solvent was dried with anhydrous sodium sulfate and spin-dried under reduced pressure. , the reaction product was purified by HPLC (methanol: water = 7: 3) to obtain a white solid intermediate (404 m...
Embodiment 2
[0034] Embodiment 2: the synthesis of compound Ib
[0035]
[0036] (1) Dissolve o-carboxybenzenesulfonic acid (360mg) in dry dimethylformamide solution (10ml) at room temperature, and add ethyl[3-(dimethylamino)propyl]carbodiethylene Amine (384mg) and 1-hydroxybenzotriazole (270mg), stirred for 15 minutes, then added L-proline benzyl ester hydrochloride (532mg) and 4-dimethylaminopyridine (805mg), the reaction solution was at room temperature The reaction was carried out for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, the reactant was diluted with dichloromethane, washed with saturated brine, 3M hydrochloric acid solution and saturated sodium chloride solution respectively, and the organic phase solvent was dried with anhydrous sodium sulfate, spin-dried under reduced pressure, and washed with The reaction product was purified by HPLC (methanol:water=5:5) to obtain a white solid product (404 mg), which was directly used in th...
Embodiment 3
[0038] Embodiment 3: the synthesis of compound Ic, Id
[0039]
[0040] (1) At room temperature, add o-sulfonamide benzoic acid (50mg, 0.25mmol) in thionyl chloride (3ml) in the reaction flask, raise the temperature of the system to reflux, react for 2 hours, remove thionyl chloride under low pressure, Dissolve the residue in dry dichloromethane, add it to a solution of L-proline benzyl ester hydrochloride (60mg, 0.25mmol) in dry dichloromethane at 0°C, and then add triethylamine (56mg, 0.55mmol), the reaction solution was reacted at room temperature for 2 hours. After the reaction was completed, the reactant was diluted with dichloromethane, washed with saturated sodium bicarbonate and saturated sodium chloride solution respectively, and the organic phase solvent was dried with anhydrous sodium sulfate, spin-dried under reduced pressure, and subjected to silica gel column chromatography (petroleum ether: ethyl acetate=2:1) to purify the reaction product to obtain the ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com