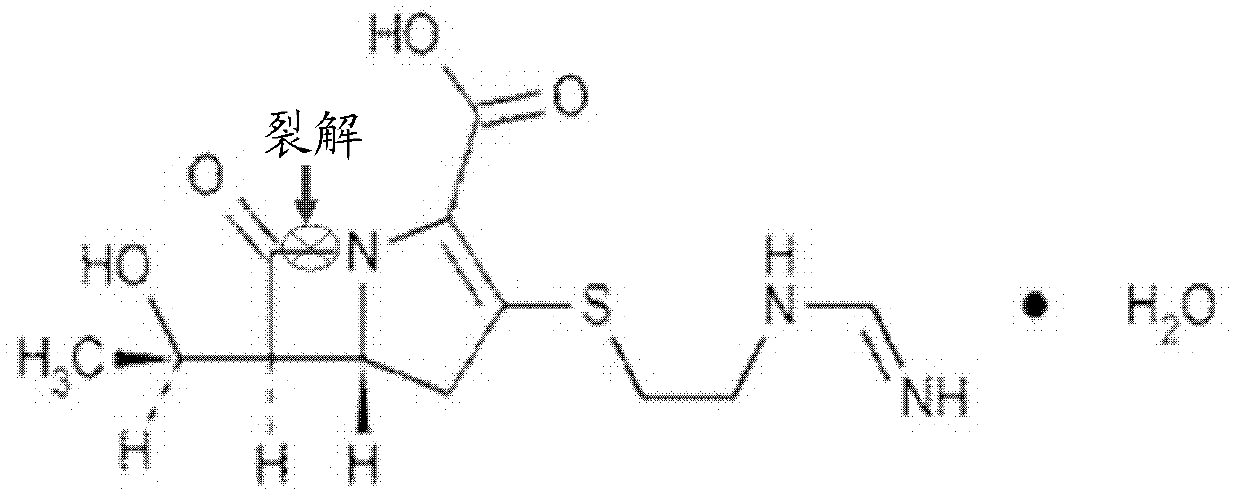
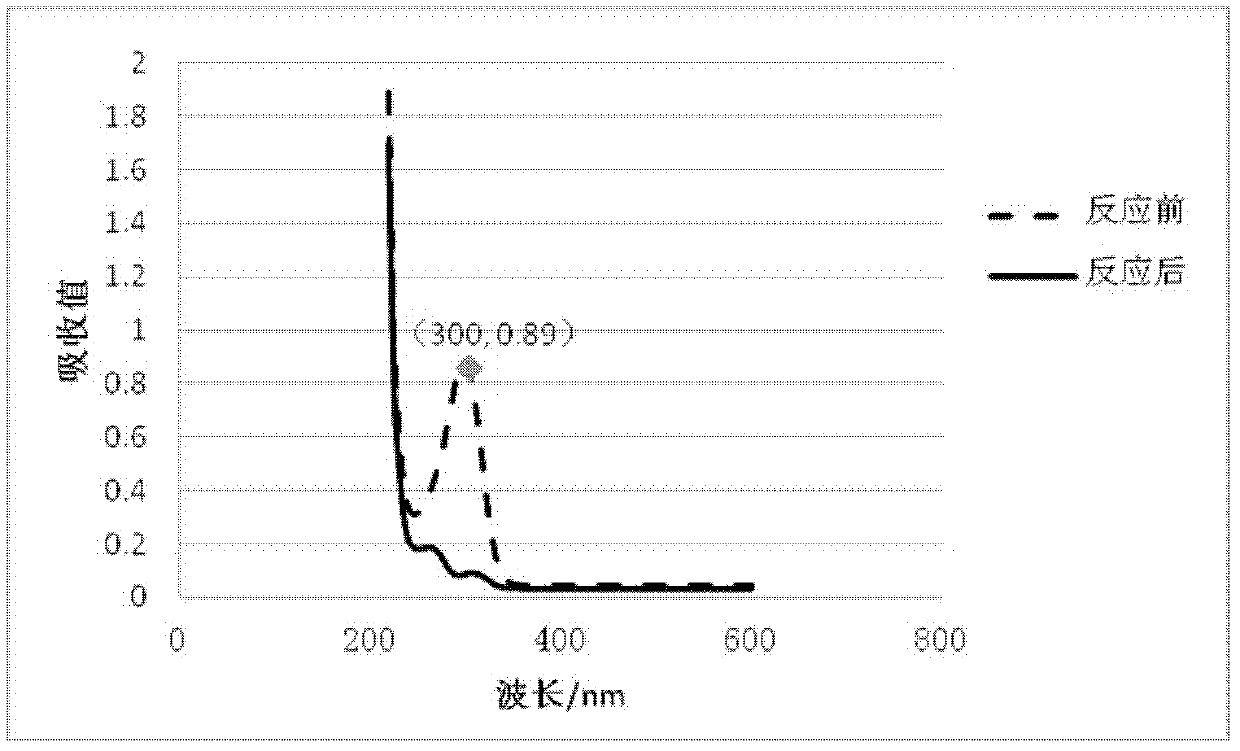
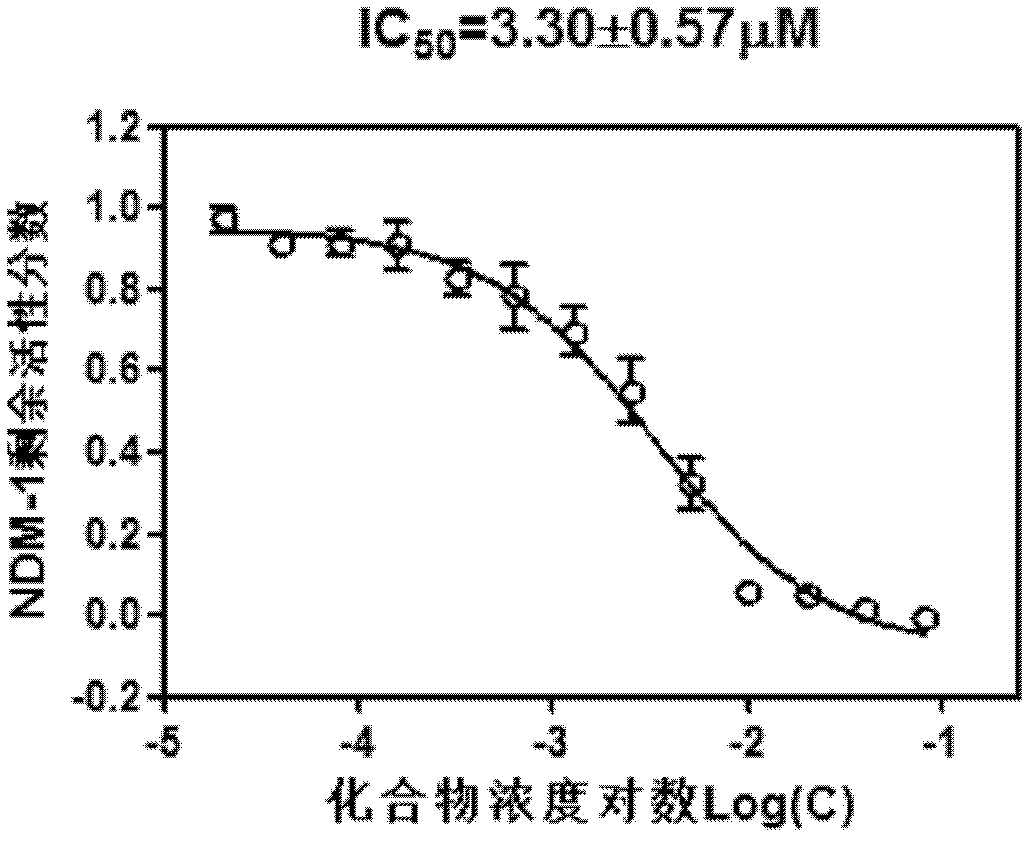
Application of isatin thiosemicarbazone compound in inhibition of NDM-1 activity
A technology of indolin diketide thiosemicarbazide and NDM-1 is applied in the directions of organic active ingredients, medical preparations containing active ingredients, antibacterial drugs, etc. problems such as the application of thiourea compounds, to achieve good drug application prospects, eliminate hydrolysis, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Compound 1
[0040]
[0041] Mix 7-iodoindolindione (2.7g, 10mmol) and phenylthiosemicarbazide (1.7g, 10mmol) with 5mL of acetic acid, reflux in 50mL of ethanol-water (5:1) solution for 6h, and then cool to At room temperature, recrystallization from ethanol solution gave 2.7 g of the title compound with a yield of 65%.
Embodiment 2
[0042] Example 2: Compound 2
[0043]
[0044] The 7-iodoindolindione in Example 1 was replaced with 7-bromoindolindione, and the compound was prepared by the same preparation method as in Example 1.
Embodiment 3
[0045] Example 3: Compound 3
[0046]
[0047] The 7-iodoindolindione in Example 1 was replaced by 7-methylindolindione, and the compound was prepared by the same preparation method as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com