Method for detecting phenolic acid substances in blood plasma
A technology for phenolic acids and plasma, applied in the field of detecting drug content in blood, can solve problems such as difficulty in separation, and achieve the effect of rapid method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the establishment of analysis method
[0056] 1 Preparation of solution
[0057] 1.1 Series standard control solution preparation
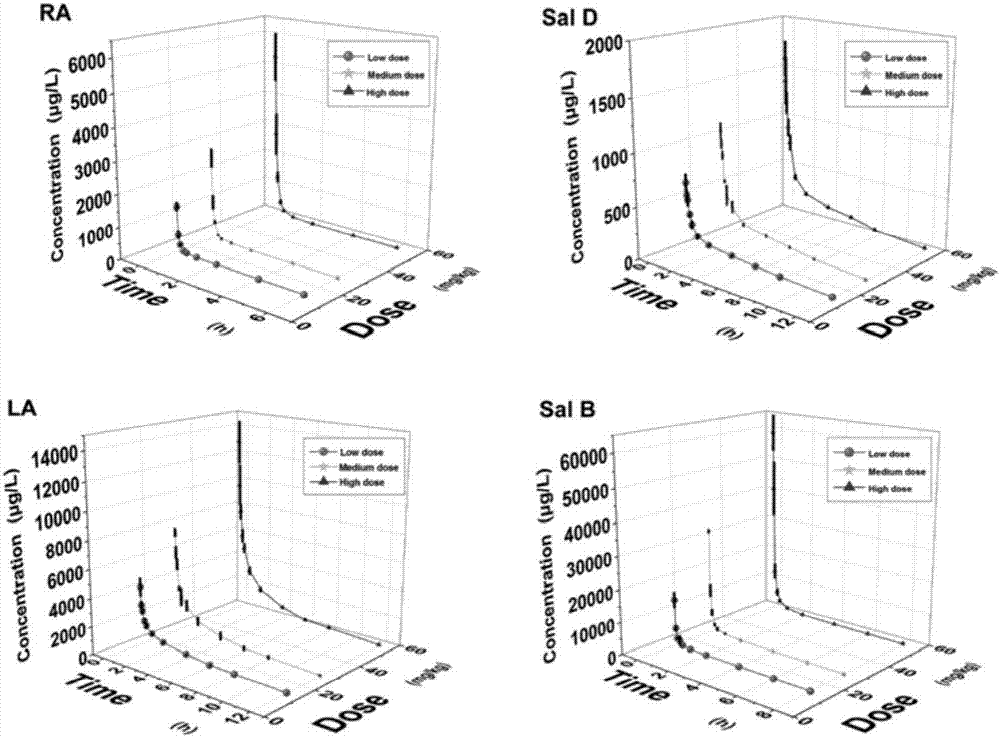
[0058] Precisely weigh 10.00 mg of rosmarinic acid, Sal D, shikonic acid, and Sal B reference substances in a 5 mL volumetric flask, dissolve them in methanol and make up to a concentration of 2.00 mg·mL -1 The stock solutions of rosmarinic acid and Sal D were diluted with methanol to concentrations of 1, 2, 5, 20, 50, 200, 500, 1000, 5000ng·mL -1 A series of standard control solutions, shikonic acid and Sal B stock solutions were diluted with methanol to concentrations of 5, 10, 25, 100, 250, 1000, 2500, 5000, and 25000 ng·mL -1 A series of standard control solutions, stored at -20°C.
[0059] 1.2 Preparation of internal standard solution
[0060] Accurately weigh 0.5mg of chloramphenicol (IS) reference substance in a 10mL volumetric flask, dissolve it with methanol and make it to volume to make a concentration of 0.05mg·...
Embodiment 2
[0070] Confirmation of embodiment 2 analytical method
[0071] 1. Mass Spectrometry
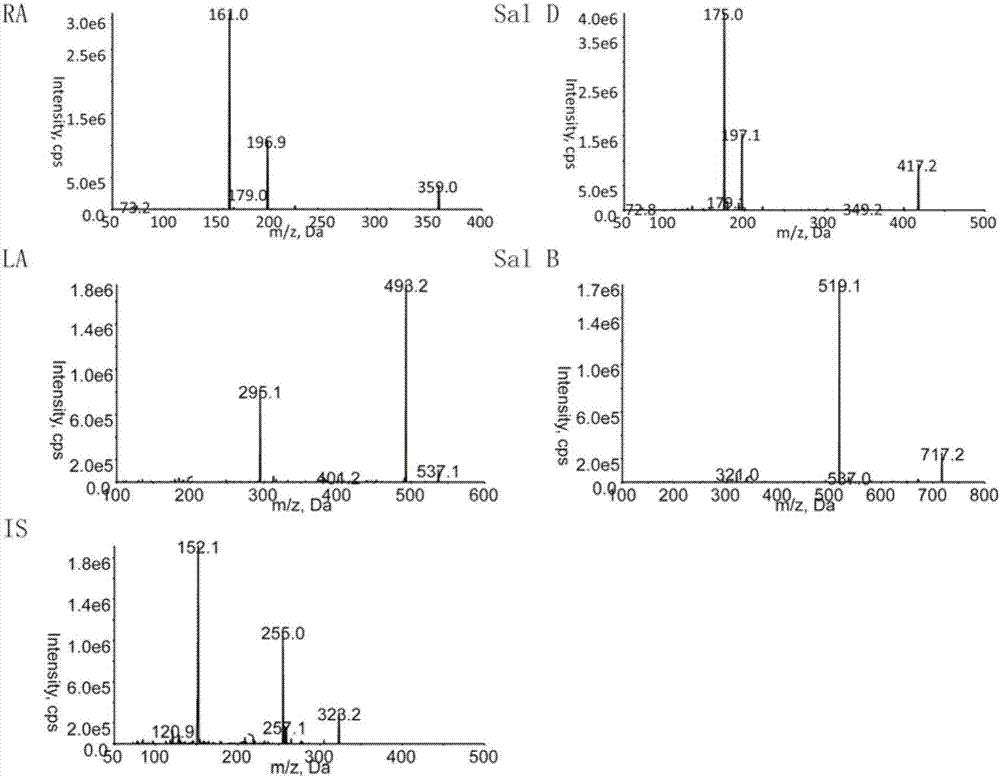
[0072] Prepare certain concentrations of rosmarinic acid, Sal D, shikonic acid, Sal B, and chloramphenicol respectively. Under ESI ionization mode, rosmarinic acid, Sal D, shikonic acid, Sal B, and chloramphenicol mainly produce [M-H ]-Quasi-molecular ion peaks are m / z359.0, m / z 417.1, m / z537.0, m / z 717.0, m / z 321.1, and the main fragment ions are m / z 161.1, m / z175.0 , m / z 493.1, m / z 519.1, m / z 152.0, see the results figure 1 .
[0073] 2. Method specificity
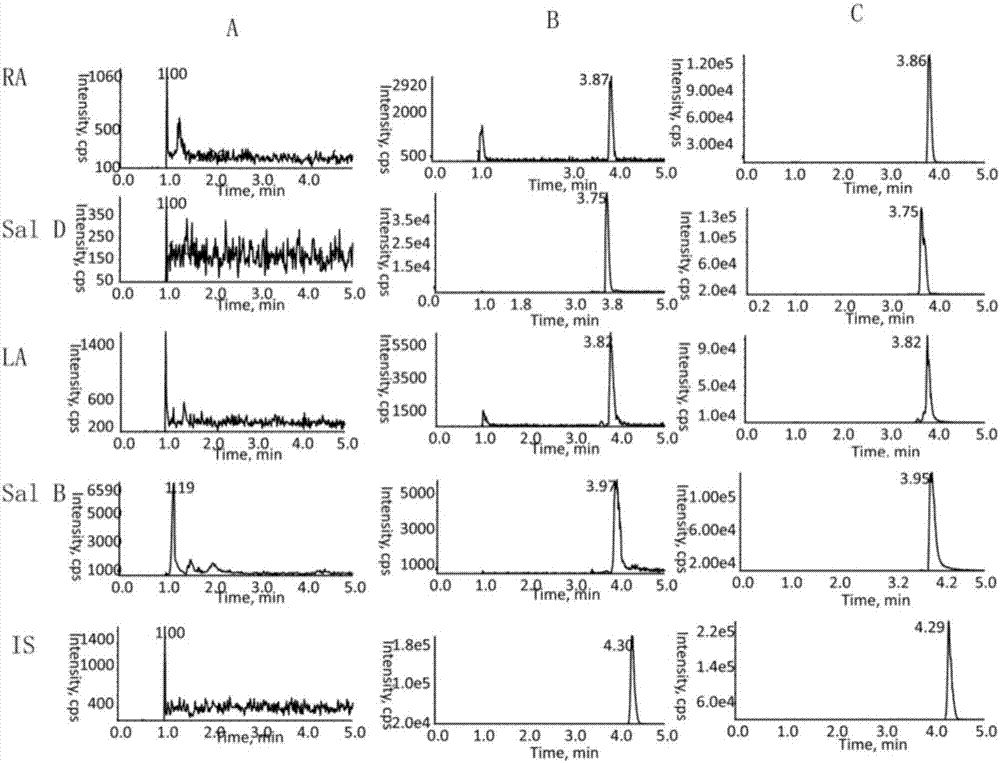
[0074] Take 100 μL of blank plasma from 6 Wistar rats in a 1.5mL centrifuge tube, replace the internal standard with 10 μL of methanol, and perform LC-MS / MS analysis according to the law under the “Plasma Sample Processing” item. For details, see figure 2 , to obtain a chromatogram of a blank plasma sample as figure 2 A: Add a certain standard solution into blank plasma and internal standard solution, and operate in the same way ...
Embodiment 3
[0097] Embodiment 3: condition optimization
[0098] 1. Column selection
[0099] This test examines Waters ACQUITY UPLC HSS T3 (1.7μm, 2.1×100mm) T3, ACQUITY UPLC HSS C 18 (1.7μm, 2.1×100mm), CORTECS TM UPLC C 18(1.6μm, 2.1×100mm) 3 chromatographic columns. Waters ACQUITY UPLC HSS T3 and CORTECS TM UPLC C 18 For the separation effect of the four compounds, the column efficiency is higher. CORTECS TM UPLC C 18 The peak position of chloramphenicol on the inner side of the chromatographic column is relatively backward, but the sample residue is more serious when using the T3 chromatographic column, so CORTECSTM UPLC C 18 As the test column.
[0100] 2. Mobile phase selection
[0101] Compared methanol and acetonitrile, it was found that when acetonitrile was used as the organic phase, the chromatographic peak shape was better and the response was higher than that of methanol. The study also found that adding 0.1% formic acid to the water phase resulted in better chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com