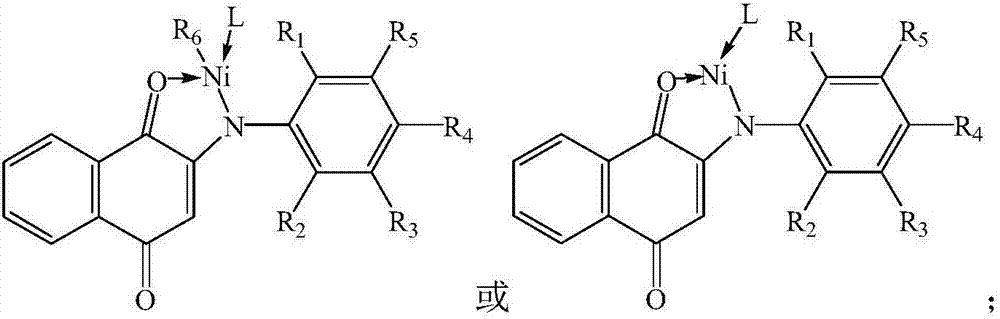
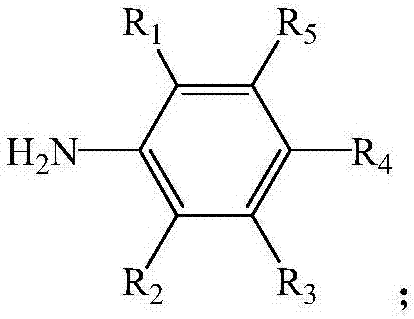
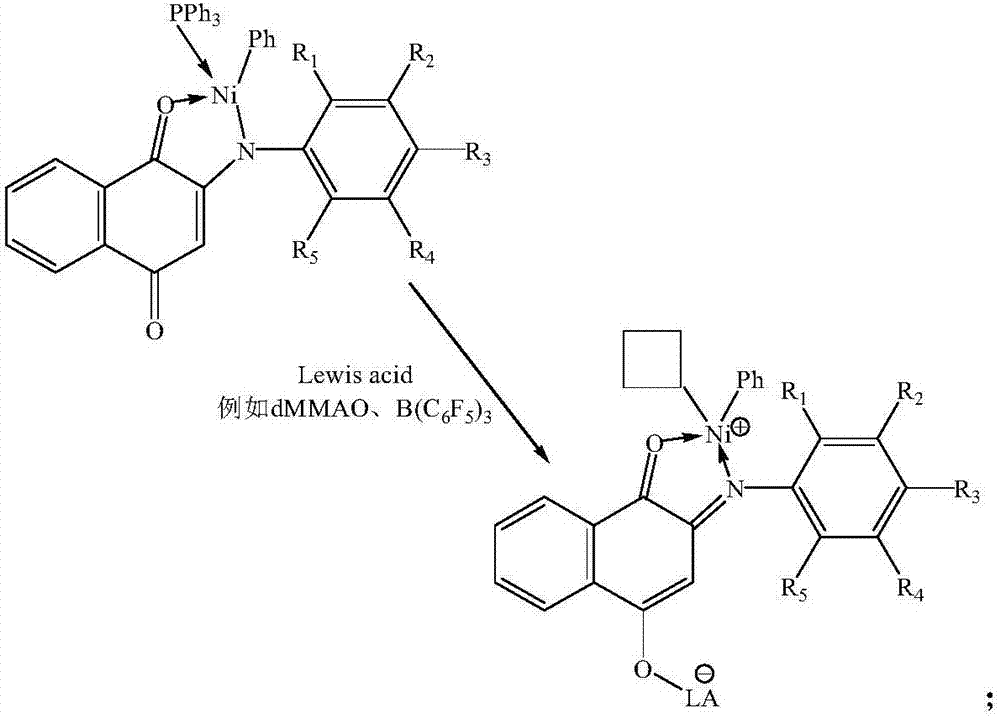
Catalyst, preparation method thereof, composition prepared from catalyst and applications of catalyst and compositions
A catalyst and reaction technology, applied in the field of olefin catalysis, can solve the problem of low catalytic activity and achieve high activity, good catalytic performance, and easy to leave
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A preparation method of catalyst, the steps are as follows:
[0065] (1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in n-heptane, add trifluoroacetic acid to react to obtain anilinonaphthoquinone ligands, the reaction temperature is 100°C, the time is 15h, the reaction Initially, the molar ratio of 2-hydroxy-1,4-naphthoquinone to substituted aniline was 1:1.15, and the molar ratio of trifluoroacetic acid to 2-hydroxy-1,4-naphthoquinone was 0.33:1,2-hydroxy- The concentration of 1,4-naphthoquinone in n-heptane is 0.125mol / L, and the structural formula of substituted aniline is as follows:
[0066]
[0067] In the formula, R 1 is methyl, R 2 is ethyl, R 3 is methyl, R 4 is ethyl, R 5 It is a methyl group, and after the reaction, the anilinaquinone ligands are purified by toluene recrystallization;
[0068] (2) Dissolving the anilino-naphthoquinone ligands in toluene, adding sodium hydride to react to obtain a ligand salt compound, the temperatu...
Embodiment 2
[0074] A preparation method of catalyst, the steps are as follows:
[0075] (1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in toluene, add trifluoroacetic acid to react to obtain aniline naphthoquinone ligands, the reaction temperature is 110°C, the time is 6h, when the reaction starts , the molar ratio of 2-hydroxyl-1,4-naphthoquinone to substituted aniline is 1:1, the molar ratio of trifluoroacetic acid to 2-hydroxyl-1,4-naphthoquinone is 0.32:1, 2-hydroxyl-1, The concentration of 4-naphthoquinone in toluene is 0.1mol / L, and the structural formula of substituted aniline is as shown in embodiment 1, and in the formula, R 1 is ethyl, R 2 is methyl, R 3 is ethyl, R 4 is methyl, R 5 After the reaction, the anilinaquinone ligands are purified by n-hexane extraction;
[0076] (2) Dissolving the anilinaquinone ligands in tetrahydrofuran, adding potassium hydride to react to obtain a ligand salt compound, the reaction temperature is 40°C, and the time is 1h. ...
Embodiment 3
[0080] A preparation method of catalyst, the steps are as follows:
[0081](1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in chlorobenzene, add trifluoroacetic acid to react to obtain anilino-naphthoquinone ligands, the reaction temperature is 130°C, the time is 6h, and the reaction starts , the molar ratio of 2-hydroxy-1,4-naphthoquinone to substituted aniline is 1:1.02, and the molar ratio of trifluoroacetic acid to 2-hydroxy-1,4-naphthoquinone is 0.32:1,2-hydroxy-1 , the concentration of 4-naphthoquinone in chlorobenzene is 0.11mol / L, and the structural formula of substituted aniline is as shown in embodiment 1, and in the formula, R 1 is isopropyl, R 2 is tert-butyl, R 3 is tert-butyl, R 4 is isopropyl, R 5 It is isopropyl, and after the reaction, the p-aniline ligands are purified by recrystallization from tetrahydrofuran;
[0082] (2) Aniline naphthoquinone ligands are dissolved in dichloromethane, and n-butyllithium is added to react to obtain a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com