Method for preparing amide impurity of ibuprofen arginine injection
A technology for injection of amide and arginine, which is applied in the field of drug synthesis, can solve the problems of poor water solubility of ibuprofen, and achieve the effects of simple and feasible process, easy-to-obtain raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 1.03g (0.005mol) ibuprofen to a 50ml one-necked flask, then add 2.98g (0.025mol) thionyl chloride, stir to dissolve at 15°C, add 1 drop of DMF dropwise, and stir at room temperature for 1h. Concentrate the excess thionyl chloride to obtain the acid chloride intermediate for use.
[0026] Add 20ml of tetrahydrofuran to the above crude product, stir and dissolve at 15°C, then add 0.87g (0.005mol) arginine, stir until all arginine is dissolved, and continue to react at room temperature for 1h. Tetrahydrofuran was concentrated under reduced pressure to obtain 1.82 g of the crude amide impurity product with a content of 80.23% and a yield of 80.57%; then the methanol solution was quickly passed through the column to obtain a methanol solution of the target product, and methanol was concentrated to obtain 1.25 g of the target product with a content of 99.1% and a yield of The rate is 68.35%.
Embodiment 2
[0028] Add 2.06g (0.01mol) ibuprofen into a 50ml one-necked flask, then add 12.7g (0.1mol) oxalyl chloride, stir to dissolve at 20°C, add 2 drops of pyridine dropwise, and stir at room temperature for 2h. Concentrate the excess oxalyl chloride to obtain the acid chloride intermediate for use.
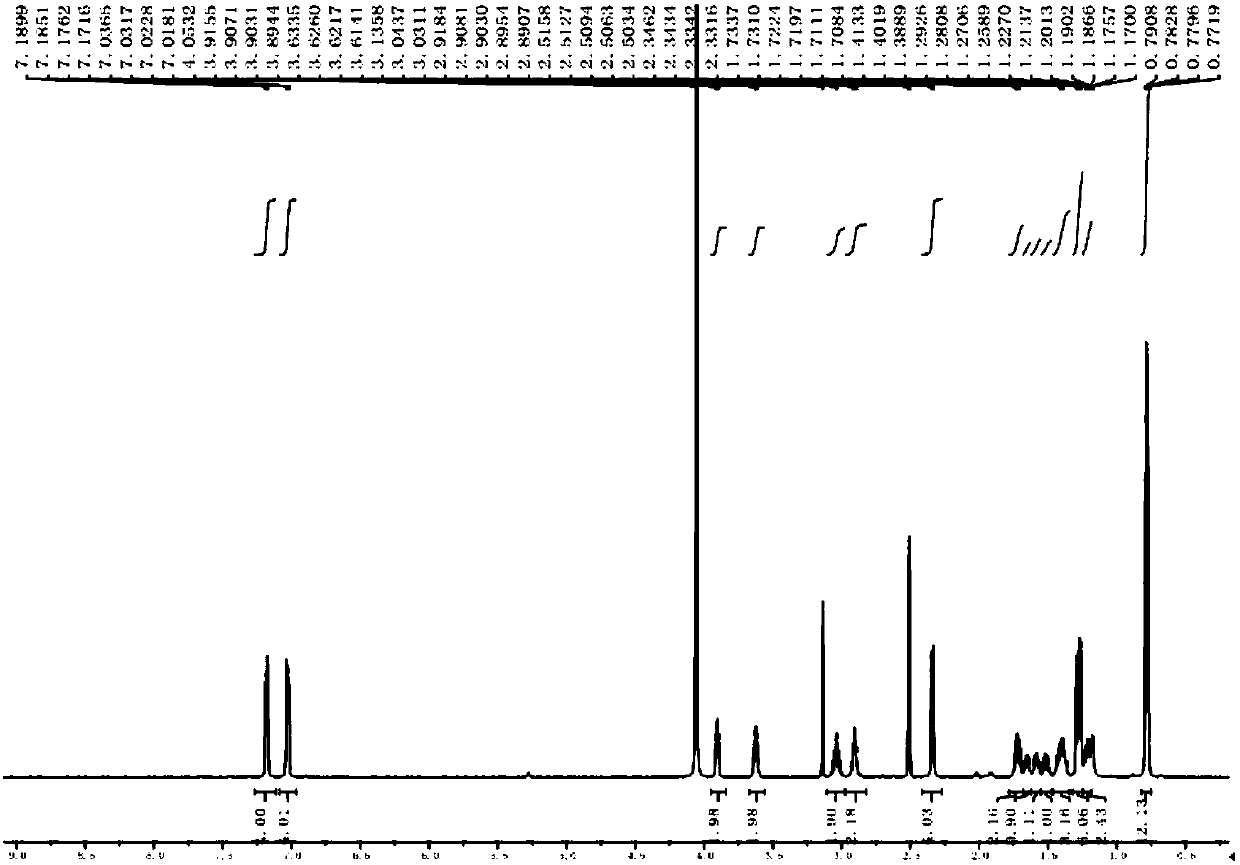
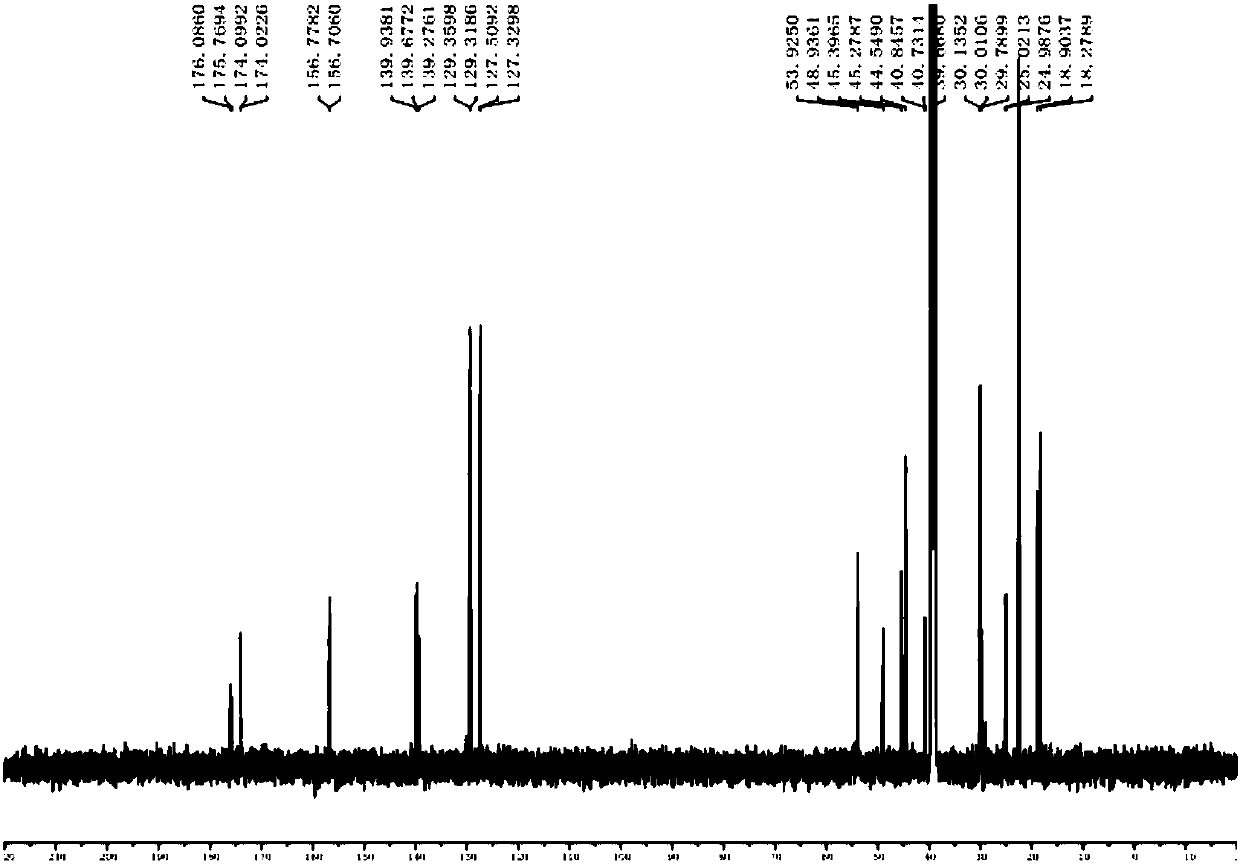
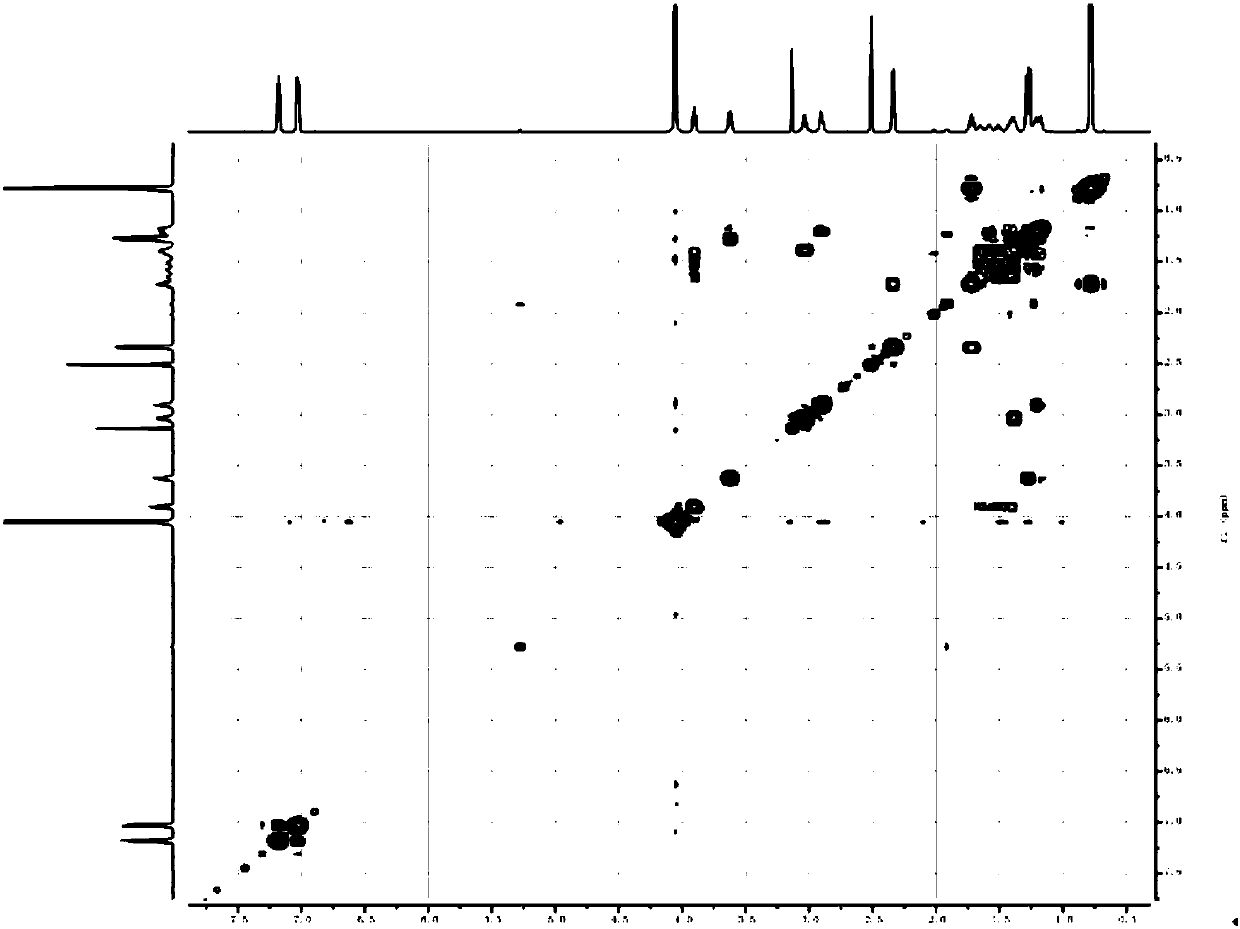
[0029] Add 20ml of DMF to the above crude product, stir to dissolve at 20°C, then add 1.83g (0.0105mol) arginine, and stir until all arginine is dissolved. Continue to react at room temperature for 2h. DMF was concentrated under reduced pressure to obtain 3.60 g of the crude amide impurity product with a content of 93.45% and a yield of 92.81%; then, the methanol solution of the target product was obtained by quickly passing through the column with methanol, and methanol was concentrated to obtain 2.93 g of the target product with a content of 99.8%. Yield is 80.67%, its structure confirms spectrogram to see attached Figure 1~6 .
Embodiment 3
[0031] Add 1.65g (0.008mol) ibuprofen into a 50ml one-necked flask, then add 18.40g (0.12mol) phosphorus oxychloride, stir to dissolve at 25°C, add 2 drops of triethylamine dropwise, and stir at room temperature for 3h. Concentrate the excess phosphorus oxychloride to obtain the acid chloride intermediate for use.
[0032] Add 20ml of acetonitrile to the above crude product, stir and dissolve at 25°C. Then add 1.53g (0.0088mol) arginine and stir until all arginine is dissolved. Continue to react for 3h. Acetonitrile was concentrated under reduced pressure to obtain 2.92g of crude amide impurity product with a content of 78.28% and a yield of 78.83%. Then, methanol was quickly passed through the column to obtain a methanol solution of the target product, and methanol was concentrated to obtain 1.88 g of the target product with a content of 98.9%. The rate is 64.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com