Icariin bone-targeting nano-liposome, and preparation method and application thereof
A nano-liposome and icariin technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem of poor absorption of icariin, unsuitable for long-term administration, etc. problems, to achieve the effect of improving solubility and absorption problems, controlled release, and good targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Icariin bone-targeted nanoliposome I
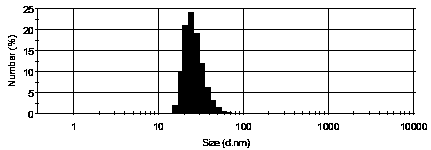
[0042] The icariin bone-targeting nanoliposome provided in this embodiment has passive bone targeting function, including icariin, soybean lecithin, cholesterol and sodium cholate, icariin, soybean lecithin, cholesterol and The mass ratio of sodium cholate is 7.5:100:20:20, and the particle size of the nanoliposome is less than 80nm, which is prepared by the following method:
[0043] Weigh 400 mg of soybean lecithin and 80 mg of cholesterol and dissolve them in an appropriate amount of absolute ethanol, and weigh 30 mg of icariin and dissolve them in absolute methanol. Mix the above components in a 250 ml eggplant-shaped bottle, evaporate under reduced pressure at 60 r / min in a water bath at 45°C to form a uniform film, add 20 ml of PBS solution (phosphate buffer) containing 80 mg sodium cholate to hydrate, and probe The ultrasonic instrument controls the temperature not to exceed 50°C, intermittent ultrasonication for ...
Embodiment 2
[0044] Example 2 Icariin bone-targeted nanoliposome II
[0045] The icariin bone-targeting nanoliposome provided in this example has passive bone targeting function, including icariin, dipalmitoylphosphatidylethanolamine, cholesterol and sodium deoxycholate, icariin, di The mass ratio of palmitoylphosphatidylethanolamine, cholesterol and sodium deoxycholate is 10:100:25:20, and the particle diameter of nanoliposomes is less than 80nm, which is prepared by the following method:
[0046] Weigh 500 mg of dipalmitoylphosphatidylethanolamine and 125 mg of cholesterol and dissolve them in an appropriate amount of absolute ethanol, and weigh 50 mg of icariin and dissolve them in absolute methanol. Mix the above components in a 250 ml eggplant-shaped bottle, evaporate under reduced pressure at 60 r / min in a 45°C water bath to form a uniform film, add 50 ml of PBS solution (phosphate buffer) containing 100 mg sodium cholate to hydrate, and probe Control the temperature of the ultrason...
Embodiment 3
[0047] Example 3 Icariin bone-targeted nanoliposomes with long circulation properties
[0048] The icariin bone-targeted nanoliposomes provided in this example have long-circulation and passive bone-targeting functions, including icariin, double-saturated lecithin, cholesterol, sodium deoxycholate and liposome surface modification The polyethylene glycol derivative (MPEG2000-DSPE), the mass ratio of icariin, double saturated lecithin, cholesterol, sodium deoxycholate and MPEG2000-DSPE is 8:100:24:10:20, nano lipid The particle size of the plastid is less than 80nm, and it is prepared by the following method:
[0049] Weigh 500 mg of double-saturated lecithin and 120 mg of cholesterol and dissolve them in an appropriate amount of absolute ethanol, weigh 40 mg of icariin and 100 mg of MPEG2000-DSPE and dissolve them in absolute methanol respectively. Mix the above components in a 500 ml eggplant-shaped bottle, evaporate under reduced pressure at 60 r / min in a water bath at 45°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com