Application of calycosin derivative in preparation of drug for treating ER feminine breast cancer
A breast cancer and drug technology, applied in the field of medicine, can solve the problems of unclear target and large effective concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of the compound shown in the formula (I) used in the following each experimental examples of the application:
[0033] Synthesized according to the following synthetic route:
[0034]
[0035] Among them, the chemical name of compound 1 is: 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one, and the chemical name of compound 2 is: 1,2-di Bromoethane, the chemical name of intermediate product 3 is: 7-(2-bromoethoxy)-3-(3-(2-bromoethoxy)-4-methoxyphenyl)-4H-benzo Pyran-4-one, the chemical name of compound 4 is: morpholine; the chemical name of compound 5 is: 3-(4-methoxy-3-(2-morpholinoethoxy)phenyl)-7 -(2-Morpholinoethoxy)-4H-benzopyran-4-one.
[0036] The specific synthesis method is:
[0037]1) Take 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one (300mg) and 1,2-dibromoethane in a molar ratio of 1:8 Dissolve in 13mL of acetone, then use triethylamine to adjust the pH of the system to 10, react at room temperature for 8h, spin...
experiment example 1
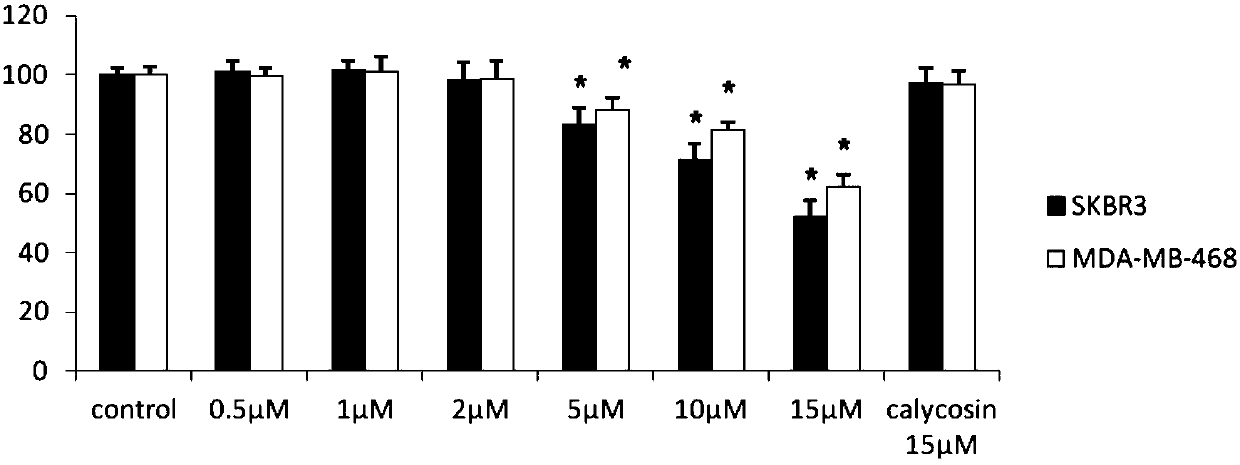
[0049] Experimental example 1: Detection of endothelial cell proliferation by MTT method
[0050] Take SKBR3, MDA-MB-468 that has reached 90% confluence, wash and digest, and make cell suspension. After counting, press 1x10 4 Each well was seeded in a 96-well plate, and cultured for 24 hours until the cells were completely adhered to the wall. In the experimental group, different concentrations of CAG002 (1 μM ~ 15 μM) were added to the groups, and each group had 9 replicate wells, and 200 μl / well of the low serum culture medium containing the drug was added. Two groups of controls were set at the same time, the blank control group was 0.1% DMSO culture solution, and the positive control was 15 μM calycosin.
[0051] MTT results showed that CAG002 could inhibit the proliferation of ER-negative breast cancer cells (SKBR3, MDA-MB-468) in a dose-dependent manner. Among them, when the drug concentration was 15 μM, the inhibitory effect was the most obvious. Compared with the bl...
Embodiment 2
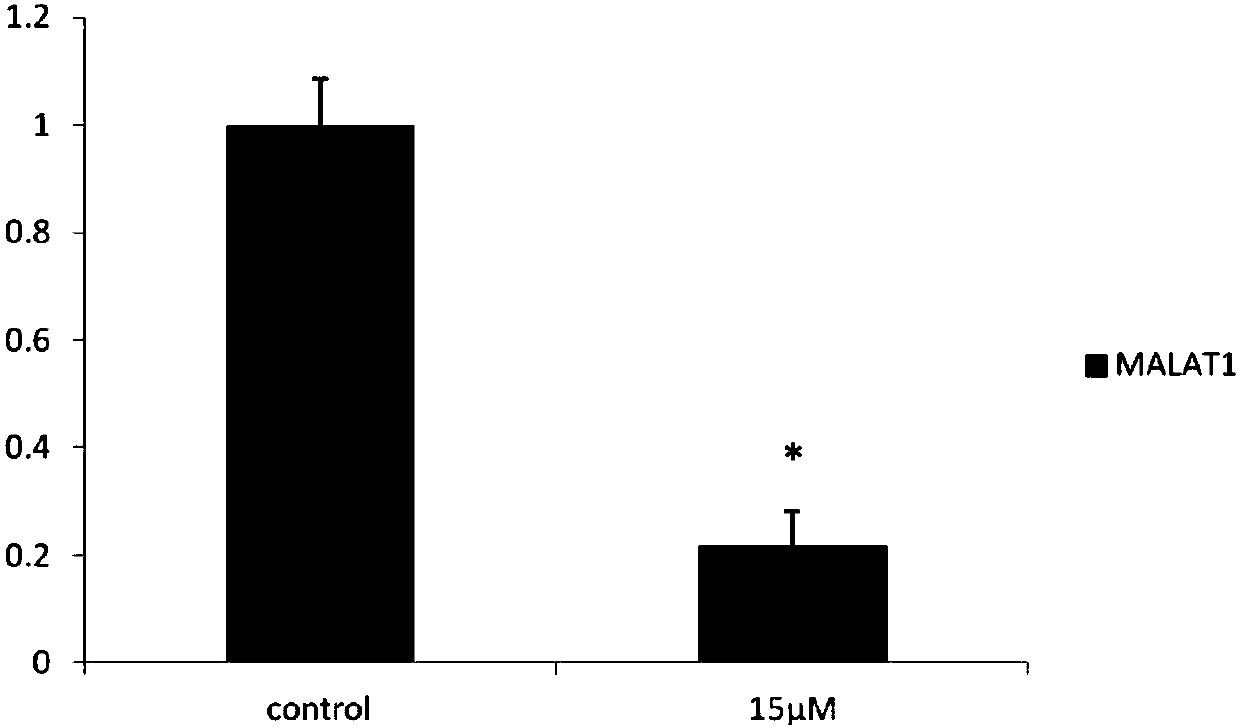
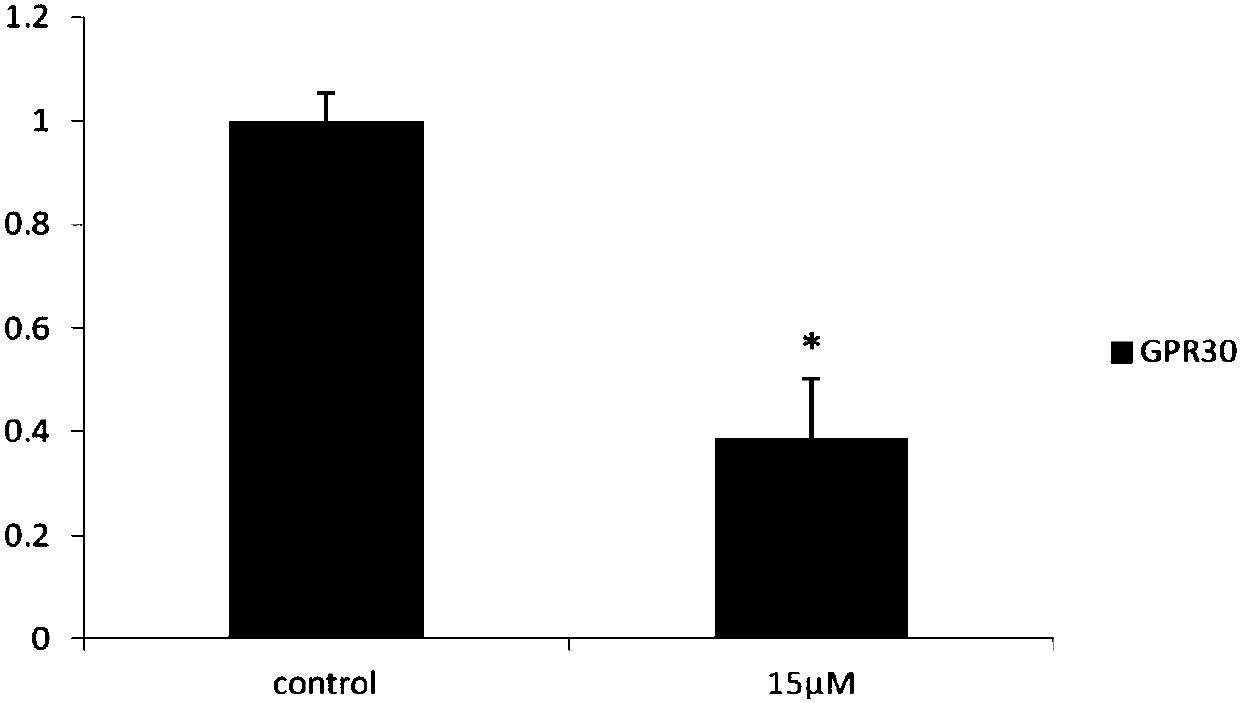
[0052] Example 2: Long-chain non-coding chip detection CAG002 affects SKBR3 gene expression in ER-negative breast cancer cells
[0053] SKBR3 cultured in 10cm 2 Petri dish to 90% confluent. After the original culture solution was removed, it was treated with a culture solution containing 15 μM CAG002 for 48 hours, and 0.1% DMSO was used as a blank control. According to the instructions of TRIZOL reagent, the total cellular RNA was extracted, and the samples were sent to Huada Genomics Company for long-chain non-coding chip detection. The drug down-regulated 116 long-chain non-coding RNAs, of which 10 long-chain non-coding RNAs such as MALAT1 were the most obvious, and MALAT1 It is 0.192 of the control group, and the results are shown in Table 1.
[0054] Table 1: Long-chain non-coding microarray detection of CAG002 affecting SKBR3 gene expression in ER-negative breast cancer cells
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com