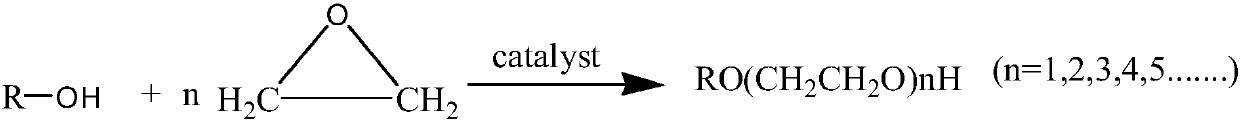
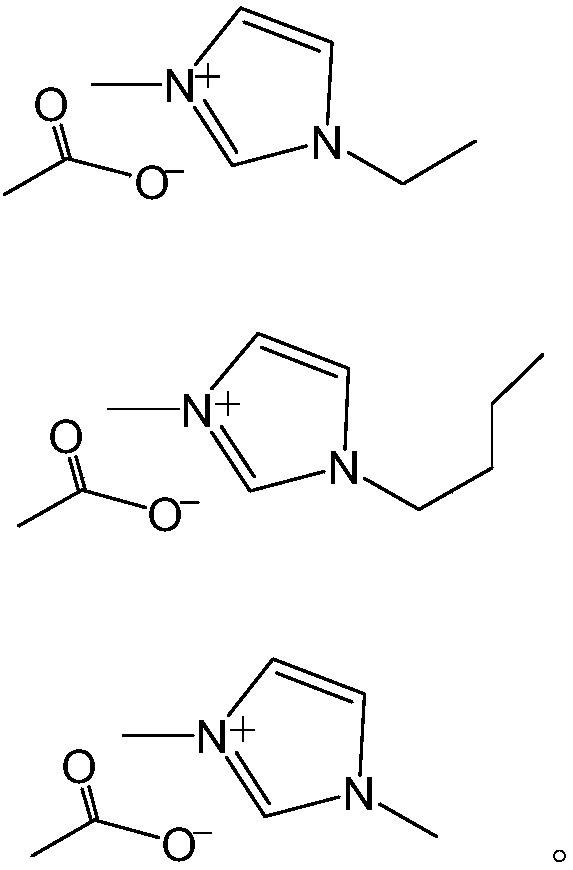
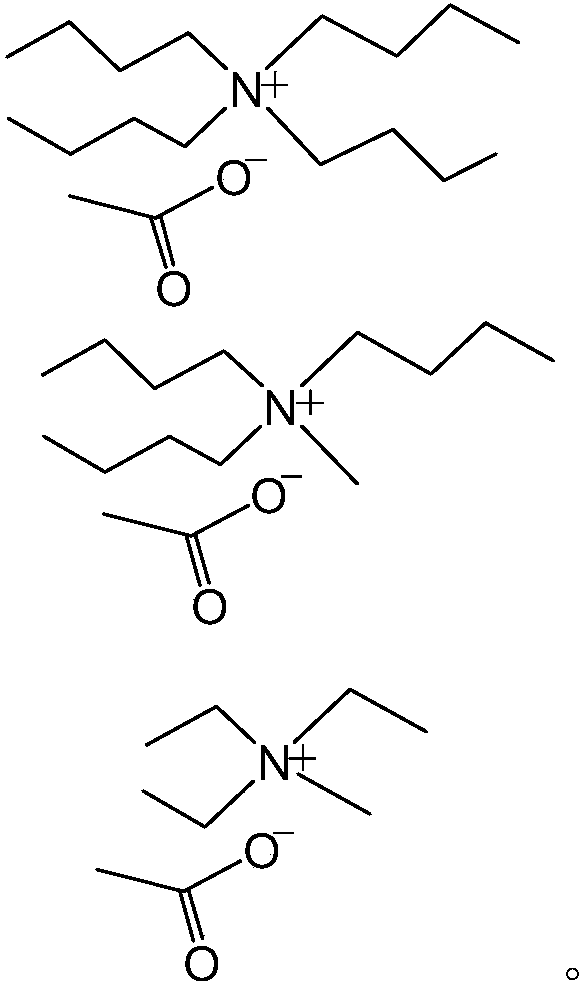
Catalyst for ethylene oxide ring-opening reaction and preparation method for oxethyl compound
An ethoxy compound and ethylene oxide technology, which is applied in the field of catalysts for ethylene oxide ring-opening reaction, can solve the problems of multiple by-products, high use cost, and few, etc., and achieves easy mixing and configuration and catalytic reaction efficiency. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of the ethoxy compound of one embodiment of the present invention comprises reacting starter and ethylene oxide under the effect of above-mentioned catalyst to prepare ethoxy compound.
[0017] In one embodiment of the present invention, the initiator can be C 1 ~C 20 Aliphatic alcohols such as methanol, ethanol, butanol, tert-butanol, octanol, dodecyl alcohol, tetradecyl alcohol, cetyl alcohol, oleyl alcohol, stearyl alcohol, allyl alcohol, methallyl alcohol, Prenyl alcohol, ethylene glycol, diethylene glycol, triethylene glycol, glycerin, etc.
[0018] In one embodiment of the present invention, the consumption of catalyst can be 0.05%~2%, such as 0.1%, 0.5%, 1% of the mass of product ethoxylate compound (the sum of reactant initiator and ethylene oxide mass) , 1.5%, etc.
[0019] In one embodiment of the present invention, according to the performance requirements of the target product ethoxylate, the reaction of different epoxide addition n...
Embodiment 1
[0023] At room temperature, add 64g of methanol and 0.05g of [EMIM][AC] into a 200mL autoclave, seal it, replace it with nitrogen three times, then raise the temperature to 60°C, then add ethylene oxide in batches, and maintain the reaction during the addition The pressure is about 0.5MPa. After adding the required 26g of ethylene oxide, continue aging for 0.5h until the reaction pressure no longer decreases, then cool down and exit the kettle. The product of gained is distilled, and ethylene glycol monomethyl ether 43g is collected, and it is compared with standard sample, and its purity is 99.2% with Agilent gas chromatographic analysis.
[0024] The still residue of distillation gained is used as catalyst, continues to repeat above reaction, can collect ethylene glycol monomethyl ether 42.3g, shows that [EMIM][AC] ionic liquid catalyst can be recycled.
Embodiment 2
[0026] At room temperature, add 74g of n-butanol and 0.2g of [BMIM][AC] into a 200mL autoclave, seal it, replace it with nitrogen three times, then raise the temperature to 90°C, then add ethylene oxide in batches, during the addition process Keep the reaction pressure at about 0.6MPa, after adding the required 35g of ethylene oxide, continue aging for 0.5h until the reaction pressure no longer drops, then cool down and exit the kettle. The product of gained is distilled, and ethylene glycol monobutyl ether 52g is collected, and it is compared with standard sample, and its purity is 99.5% with Agilent gas chromatographic analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com