Ornidazole purity standard substance and preparation method and application thereof
A standard substance, ornidazole technology, applied in the field of standard value transfer in stoichiometry, can solve problems such as human health hazards, and achieve the effect of ensuring traceability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
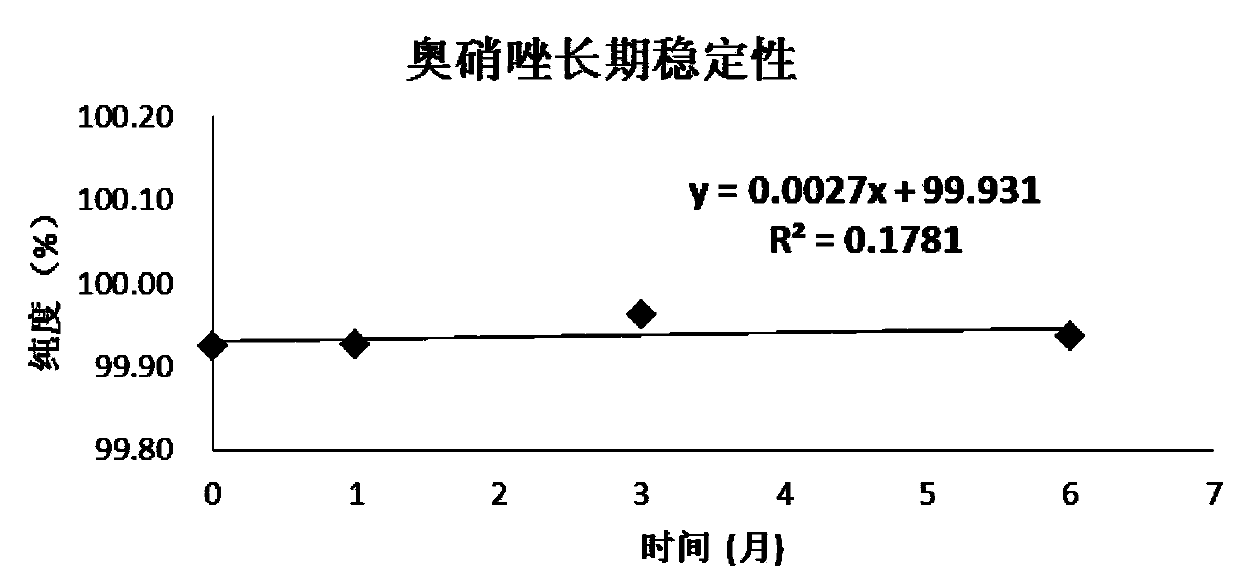
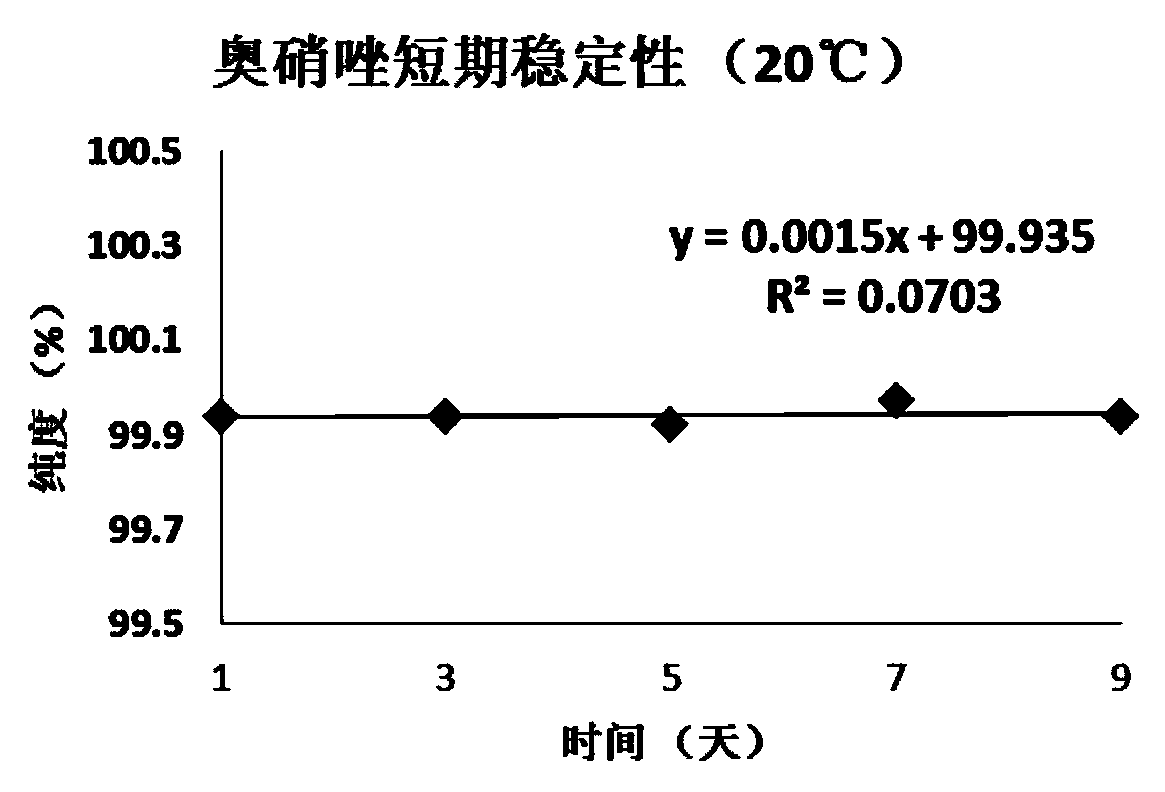
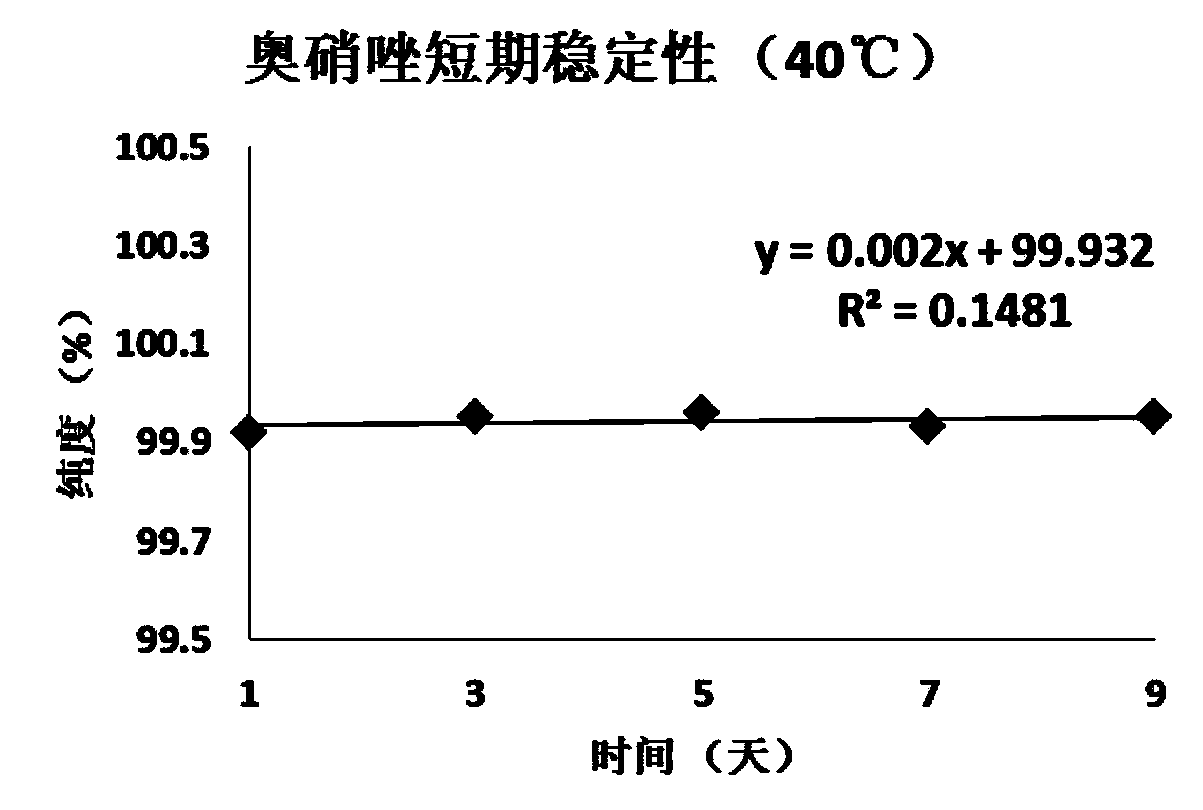
[0059] Embodiment 1, the definite value of ornidazole purity standard substance
[0060] The definite value step of ornidazole purity standard substance of the present invention is as follows:
[0061] 1. Uniformity test
[0062] According to the requirements of JJG 1006-1994 "Technical Specifications for Primary Reference Materials", the homogeneity test was carried out for the standard materials. The present invention adopts the variance analysis method to statistically check the uniformity of the ornidazole purity standard substance, randomly extracts 15 packages from the subpackaged ornidazole, each package is taken in parallel three times, and the weight-volume method is used to prepare 100mg / L methanol solution, Using the conditions when the value was fixed, liquid chromatography-area normalization method inspection, the obtained uniformity data and statistical results are shown in Table 1.
[0063] Table 1 ornidazole purity standard substance homogeneity inspection re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com