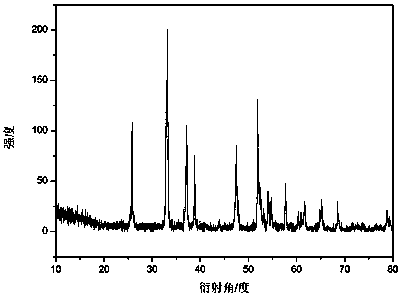
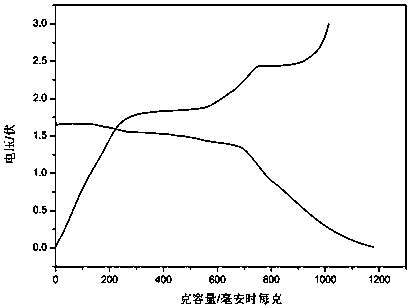
Preparation method of flaky nanometer FeS2/C negative electrode material
A negative electrode material and nanotechnology, which is applied in the field of preparation of sheet-like nano FeS2/C negative electrode materials for lithium ion batteries, can solve the problems of low discharge capacity of electrode materials, and achieve uniform product shape and size, high ion transmission speed, The effect of improving ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment includes the following steps:
[0034] (1) Dissolve 4 mmol of fumaric acid and 4 mmol of ferric chloride hexahydrate in 40 mL of deionized water, and stir for 1 hour to obtain a homogeneous solution.
[0035] (2) Put the homogeneous solution obtained in step (1) into a 100 mL stainless steel reaction kettle lined with polytetrafluoroethylene, put it in a drying oven, and conduct a hydrothermal reaction at 120°C for 15 hours, then naturally cool to room temperature , filtered, washed the precipitate four times with absolute ethanol and deionized water, and dried in an oven at 60°C for 24 h to obtain 1.0 g of yellow powder.
[0036] (3) Disperse 1.0g of yellow powder obtained in step (2) and 4.0g of thioacetamide in 60ml of ethanol solution; place the resulting solution in a 100 mL stainless steel reactor lined with polytetrafluoroethylene, and put it in a dry The hydrothermal reaction was carried out at 180 °C for 12 h in the oven, then naturally cooled ...
Embodiment 2
[0044] This embodiment includes the following steps:
[0045] (1) Dissolve 8 mmol of trimesic acid and 4 mmol of ferric nitrate nonahydrate in 60 mL of N,N-dimethylformamide, and stir for 1 hour to obtain a homogeneous solution.
[0046] (2) Put the homogeneous solution obtained in step (1) into a 100 mL stainless steel reaction kettle lined with polytetrafluoroethylene, put it in a drying oven, and conduct a hydrothermal reaction at 150°C for 10 hours, then cool it naturally to room temperature , filtered, washed the precipitate four times with absolute ethanol and deionized water, and dried in an oven at 60 °C for 12 h to obtain a red powder.
[0047] (3) Disperse 1.0 g of the red powder obtained in step (2) and 4.0 g of thioacetamide in 60 ml of ethanol solution, place the resulting solution in a 100 mL stainless steel reactor lined with polytetrafluoroethylene, and put it in a dry The hydrothermal reaction was carried out at 120 °C for 16 h in the oven, then naturally coo...
Embodiment 3
[0054] This embodiment includes the following steps:
[0055] (1) Dissolve 2mmol of terephthalic acid and 4mmol of ferric nitrate nonahydrate in 60mL of N,N-dimethylformamide, and stir for 1 hour to obtain a homogeneous solution.
[0056] (2) Put the homogeneous solution obtained in step (1) into a 100 mL stainless steel reaction kettle lined with polytetrafluoroethylene, put it in a drying oven, and conduct a hydrothermal reaction at 150°C for 10 hours, then cool it naturally to room temperature , filtered, washed the precipitate four times with absolute ethanol and deionized water, and dried in an oven at 60 °C for 12 h to obtain a yellow powder.
[0057] (3) Disperse 1.0g of yellow powder obtained in step (2) and 4.0g of thioacetamide in 60ml of ethanol solution; place the resulting solution in a 100 mL stainless steel reactor lined with polytetrafluoroethylene, and put it in a dry The hydrothermal reaction was carried out at 120 °C for 16 h in the oven, then naturally coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com