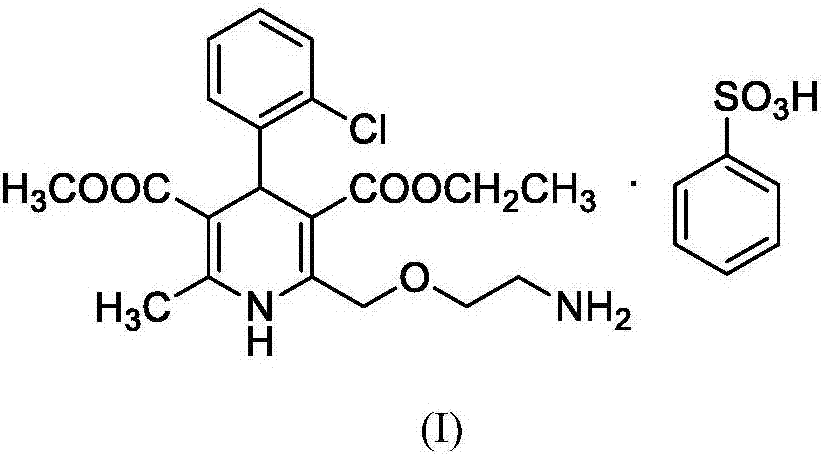
Synthesis method of amlodipine besylate degradant impurities
A kind of technology of amlodipine besylate and synthesis method, applied in the field of medicinal chemistry, can solve the problems such as compound synthesis methods that are not reported in the literature, and achieve the effects of improving quality standards, simple process, and easy availability of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 compound 1
[0033] Benzyl acetoacetate (25g), aqueous ammonia (75mL) and methanol (50mL) were added to 250mL, and stirred overnight at room temperature; TLC showed that the reaction was complete; after most of the methanol and aqueous ammonia were spinned out at room temperature, ethyl acetate (250mL) and water ( 100mL) was separated, the aqueous phase was extracted twice with ethyl acetate (100mL), the combined organic phase was washed with saturated brine, dried, and spin-dried to obtain compound 1 (light yellow oil, 25.0g), which could be used directly in Next reaction.
Embodiment 2
[0034] The preparation of embodiment 2 compound 2
[0035] SM2 (25.0g, 60% purity), o-chlorobenzaldehyde (6.6g) and isopropanol (100ml) were added in the reaction flask, piperidine (0.5mL) and acetic acid (0.75mL) were added respectively, and the temperature was raised to 40 degree, reacted overnight; TLC showed that the reaction was complete, and spin-dried after cooling down, then added ethyl acetate (400mL) to dissolve, washed with water (100mL) and saturated brine (100mL) respectively, and dried and spin-dried to obtain a yellow oil; After adding isopropanol (100mL) to the oil to dissolve, compound 1 (9.0g) was added, and the temperature was raised to 75°C to react overnight; TLC showed that after the reaction was complete, the temperature was lowered and spin-dried, and purified by column to obtain 10g of crude compound 2. The crude compound was added into 50 mL of methanol and stirred for 30 minutes, filtered and dried to obtain pure compound 2 (4.0 g, pale yellow solid)...
Embodiment 3
[0036] The preparation of embodiment 3 compound 3
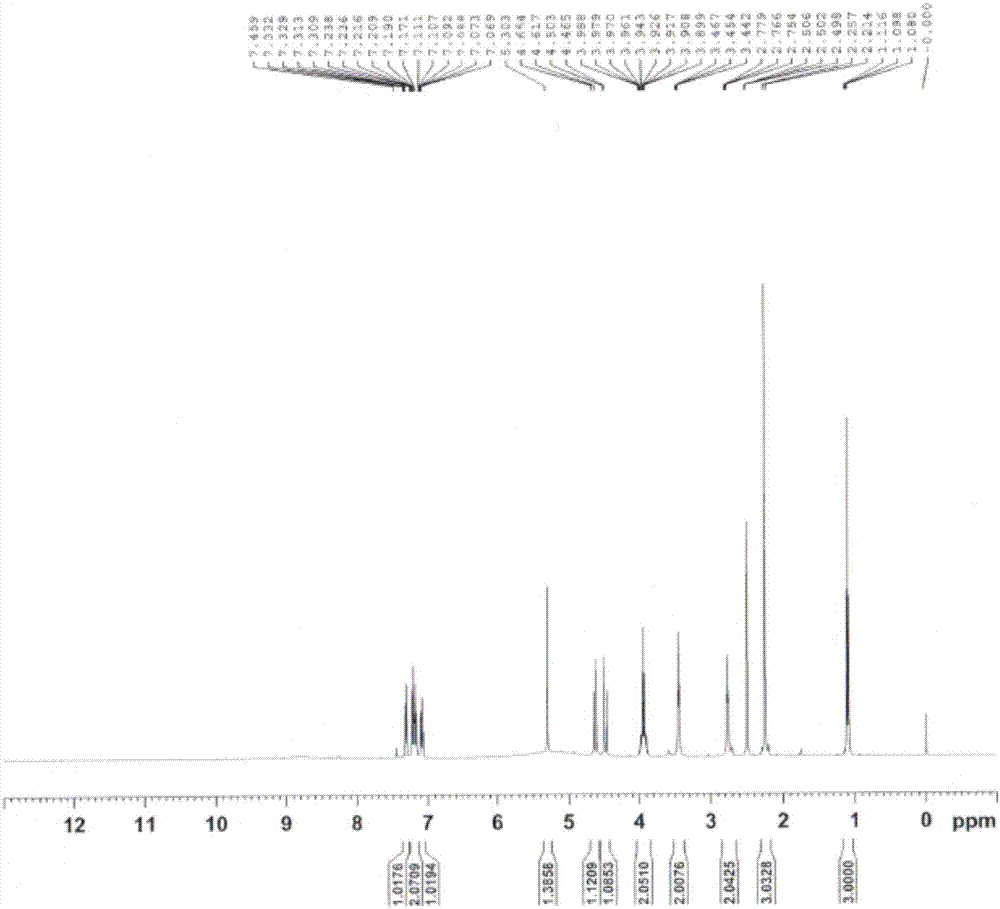
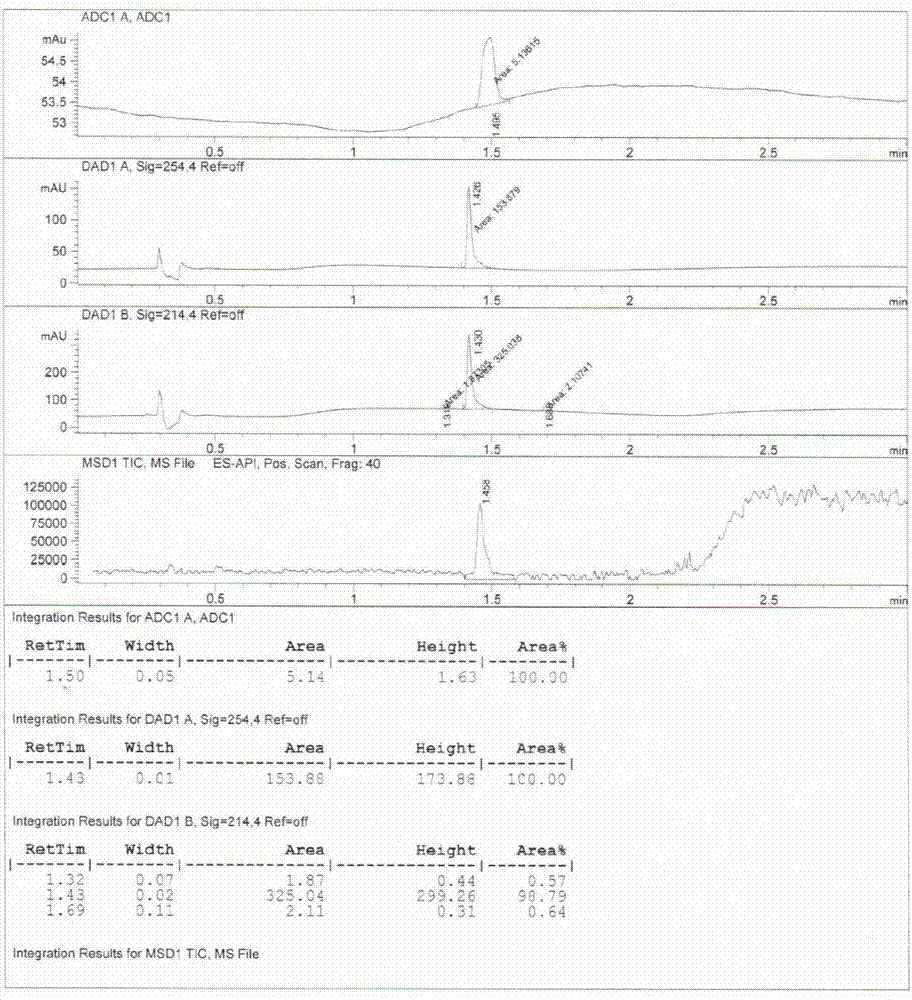
[0037] Add compound 2 to a mixed solvent of THF (150mL) and methanol (50mL), add Pd / C (500mg) to replace hydrogen, and react overnight at 25°C; filter and spin dry to obtain a solid, and add ethyl acetate to the above solid After the ester (20 mL) and dichloromethane (20 mL) were dissolved, petroleum ether (150 mL) was added, followed by crystallization, filtration, and drying to obtain compound 3 (2.0 g, white solid).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com