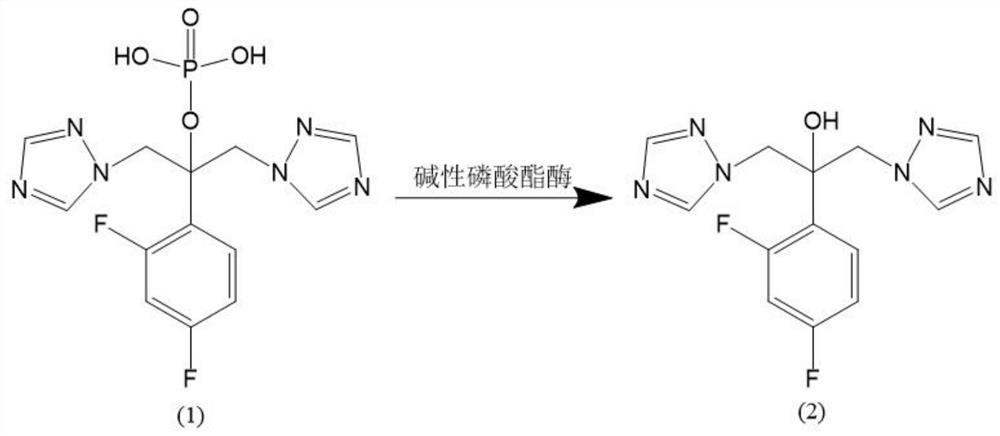
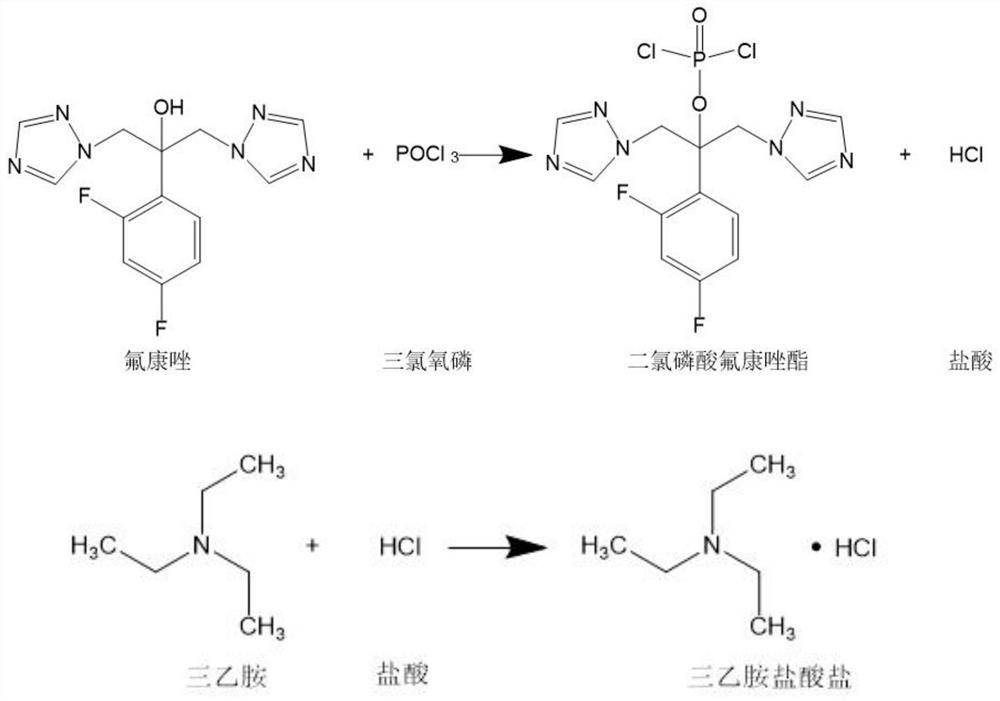
A kind of preparation method of forsefluconazole
A technology for forsifluconazole and fluconazole, applied in the field of drug synthesis, can solve the problems of low yield, many organic wastes, environmental pollution and the like, and achieve the effects of improving purity, improving yield and reducing waste of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0069] Group 1-1
[0070] Under nitrogen protection, the temperature was controlled at -10 °C, and 76.7 g of phosphorus oxychloride was slowly added dropwise to 300 ml of 3.33 mol / L triethylamine in dichloromethane solution. After dropping, the temperature was maintained at -10 °C and stirred for 1 hour. 1L of 0.5mol / L fluconazole in dichloromethane solution was added dropwise, TLC monitoring (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution) was completed; the temperature was maintained at -10°C, 650ml of water was added and stirred for 30 minutes, Sodium hydroxide was added until the pH of the aqueous phase was 10, the layers were left to stand, the upper aqueous phase was separated, and the organic phase was evaporated under reduced pressure to remove the solvent to obtain solid fluconazole dichlorophosphate.
[0071] Group 1-2
[0072] Under nitrogen protection, the temperature was controlled at -5 °C, and 161.0 g of phosphorus oxychloride was slowly added dr...
experiment example 2
[0087] Group 3-1
[0088] Under nitrogen protection, the temperature was controlled at 0 °C, and 50.6 g of phosphorus oxychloride was slowly added dropwise to 200 ml of 3.3 mol / L triethylamine in dichloromethane solution. After dropping, the temperature was maintained at 0 °C and stirred for 1 hour. 600ml of 0.5mol / L fluconazole solution in dichloromethane, monitored by TLC (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution), the reaction was completed; maintain the temperature at 0°C, add 400ml of water and stir for 30 minutes, then add hydroxide Potassium to the pH of the aqueous phase is 8, stand for stratification, separate the upper aqueous phase, and evaporate the organic phase to remove the solvent under reduced pressure to obtain solid fluconazole dichlorophosphate.
[0089] Group 3-2
[0090] Under nitrogen protection, the temperature was controlled at 0 °C, and 50.6 g of phosphorus oxychloride was slowly added dropwise to 200 ml of 3.3 mol / L triethylamin...
experiment example 3
[0093] Group 4-1
[0094] Under nitrogen protection, the temperature was controlled at 0 °C, and 50.6 g of phosphorus oxychloride was slowly added dropwise to 200 ml of a 3.3 mol / L pyrimidine solution in dichloromethane. After dropping, the temperature was maintained at 0 °C and stirred for 1 hour, and 0.5 mol of / L fluconazole in dichloromethane solution 600ml, TLC monitoring (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution) the reaction is complete; maintain the temperature at 0 ℃, add 400ml water and stir for 30 minutes, add sodium hydroxide to The pH of the aqueous phase is 8, and the layers are left to stand, the upper aqueous phase is separated, and the organic phase is evaporated under reduced pressure to remove the solvent to obtain solid fluconazole dichlorophosphate.
[0095] Group 4-2
[0096] Under nitrogen protection, the temperature was controlled at 0 °C, and 50.6 g of phosphorus oxychloride was slowly added dropwise to 200 ml of a dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com