Iron-based hydrogen production electric catalyst and preparation method
An electrocatalyst and catalyst technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problem that elemental iron easily corrodes iron oxides, reduces activity, and hydrogen evolution High overpotential problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
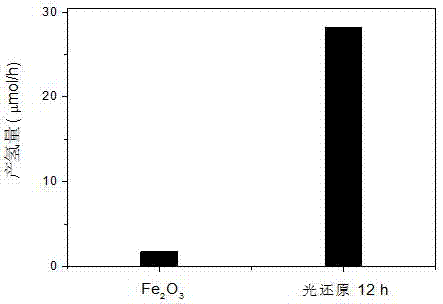
[0021] Example 1: Fe@Fe 2 o 3 Photochemical reduction preparation of catalysts and dye-sensitized photocatalytic hydrogen production performance.
[0022] (1) Preparation of Fe by microwave method 2 o 3 . 0.24 g FeCl 3 , 9 g urea, 0.014 g PEG-4000 (polyethylene glycol), dissolved in 150 mL deionized water. Place in a 500 mL three-necked flask, and heat in a microwave at 630 W for 10 min. Cool to room temperature, wash with water 5 times after centrifugation, and dry at 60°C. Reddish-brown Fe can be obtained after grinding 2 o 3 .
[0023] (2) Preparation of Fe@Fe by photochemical reduction 2 o 3 . Take 10~50 mg of Fe prepared above 2 o 3 Place in a 100 mL solution consisting of 95 mL of trimethylamine solution at pH=11 (volume ratio of trimethylamine to water=1:49) and 5 mL of a concentration of 1.0×10 -3 mol / L eosin solution mixed. Sonicate for 10 min to make Fe 2 o 3 Evenly dispersed in the solution. The solution was put into a 150 mL Pyrex light bottle, ...
Embodiment 2
[0027] Example 2: Fe@FeB x Photochemical reduction preparation of electrocatalysts and electrocatalytic hydrogen production performance.
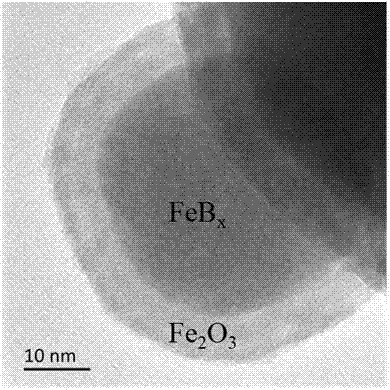
[0028] (1) Fe 2 o 3 @FeB x preparation. Dissolve 0.15 g of ferrous sulfate in 50 mL of deionized water. Dissolve 0.3 g of sodium borohydride in 20 mL of deionized water. Under stirring, the sodium borohydride solution was dropped into the ferrous sulfate solution at a rate of 0.02 mL / s, and a fluffy precipitate was formed immediately. After the dropwise addition, suction filtration was performed, and the solid phase was washed three times with water, ethanol, and acetone successively, and then dried with nitrogen or argon to obtain Fe 2 o 3 @FeB x . figure 2 For the prepared Fe 2 o 3 @FeB x Transmission electron microscopy (TEM) image. It can be seen that the shell Fe 2 o 3 The thickness is about 7 nm.
[0029] (2) Fe@FeB x Prepared by photochemical method. Take 10~50 mg of Fe 2 o 3 @FeB x Place in a 100 mL solution co...
Embodiment 3
[0037] Example 3: Fe@FeB x Electrochemical preparation and electrocatalytic performance of electrocatalysts.
[0038] (1) Fe 2 o 3 @FeB x The preparation is the same as step (1) in Example 2.
[0039] (2) FeB x preparation.
[0040] pure phase FeB x Prepare according to step (1), but the whole process is carried out in an argon-filled glove box, and dry naturally in the glove box to obtain gray FeB x .
[0041] (3) Reduced iron powder.
[0042] Analytical pure reduced iron powder was purchased from Shanghai Yingyuan Chemical Co., Ltd.
[0043] (4) Preparation of Fe@FeB by electrochemical reduction x .
[0044] Will Fe 2 o 3 @FeB x Preparation of Fe@FeB by electrochemical reduction at an applied potential of -1.853 V (electrode potential relative to saturated calomel) in a two-liquid system electrolytic cell x .
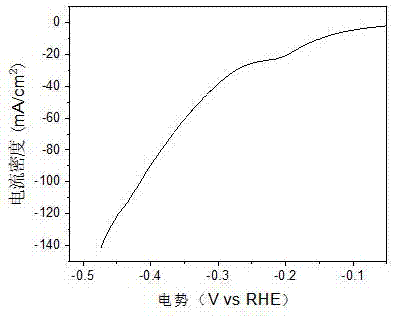
[0045] (5) Fe@FeB x , reduced iron powder and pure phase FeB x Electrocatalytic hydrogen production performance.
[0046] Fe@FeB x The working ele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com