Polyamic acid, polyimide and preparation and application thereof
A technology of polyamic acid and polyimide, which is applied in semiconductor/solid-state device manufacturing, coating, electrical components, etc., can solve the problems of high price and insufficient flexibility, and achieve good flexibility, strong hydrophobicity, and low dielectric strength. The effect of the electric constant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] In the preparation method of polyamic acid of the present invention, there is no other special requirement to the selection of aromatic tetracarboxylic dianhydride, can realize above-mentioned polycondensation reaction and get final product. In a preferred example of the present invention, the aromatic tetracarboxylic dianhydride is selected from pyromellitic dianhydride (PMDA), 3,3',4,4'-benzophenone tetracarboxylic dianhydride (BTDA ), 4,4'-diphenyl ether dianhydride (ODPA) and 4,4'-(hexafluoroisopropylene) diphthalic anhydride (6FDA).
[0064] For avoiding the oxidative degradation of polycondensation product when high temperature, to guarantee the molecular weight of product, above-mentioned polycondensation reaction preferably carries out under inert atmosphere condition. For example, it is performed under a nitrogen atmosphere.
[0065] In a preferred example of the embodiment of the present invention, the preparation of the aromatic diamine represented by the fo...
Embodiment 1
[0102] The preparation of the aromatic diamine represented by formula (III):
[0103] The preparation process is shown in the following formula:
[0104]
[0105] Its specific preparation comprises the following steps:
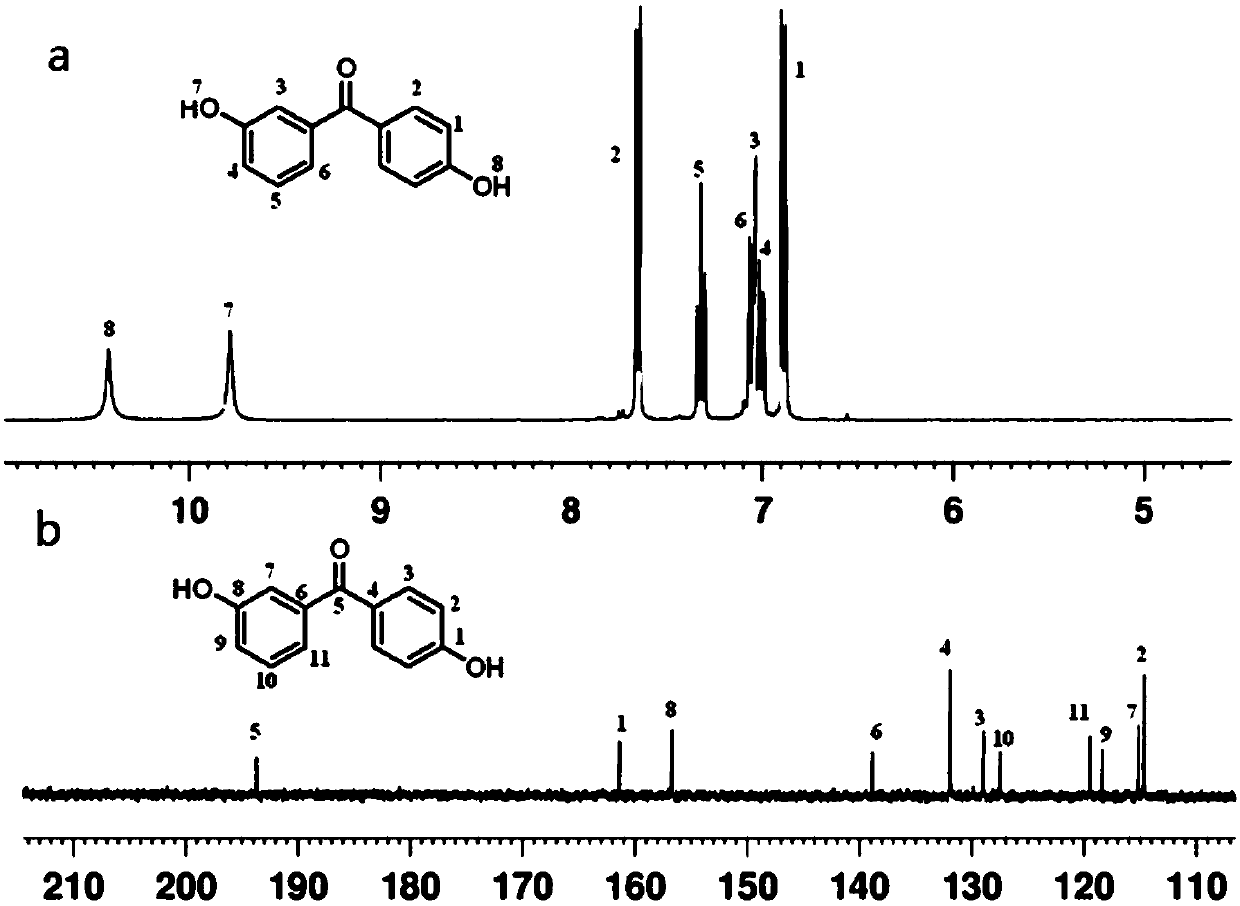
[0106] The synthesis of 3,4'-dihydroxybenzophenone represented by formula (IV):
[0107] 21.6g (0.2mol) 3-methoxybenzoyl chloride, 34g (0.2mol) anisole and 300mL CH 2 Cl 2Put into a 500mL three-necked round bottom flask equipped with magnetic stirring. After the mixture was stirred at 0-5°C in an ice bath for 0.5 hours, the temperature of the system was kept at a temperature of 0-5°C, and 40g (0.30mol) of anhydrous AlCl was mixed under the condition of magnetic stirring 3 Slowly add CH 2 Cl 2 In the process, the addition is completed in 1.5 hours, and the temperature must be strictly controlled during this process. The mixture was then poured into water, the organic phase was extracted from the water, and the solvent was evaporated to dryness by rota...
Embodiment 2
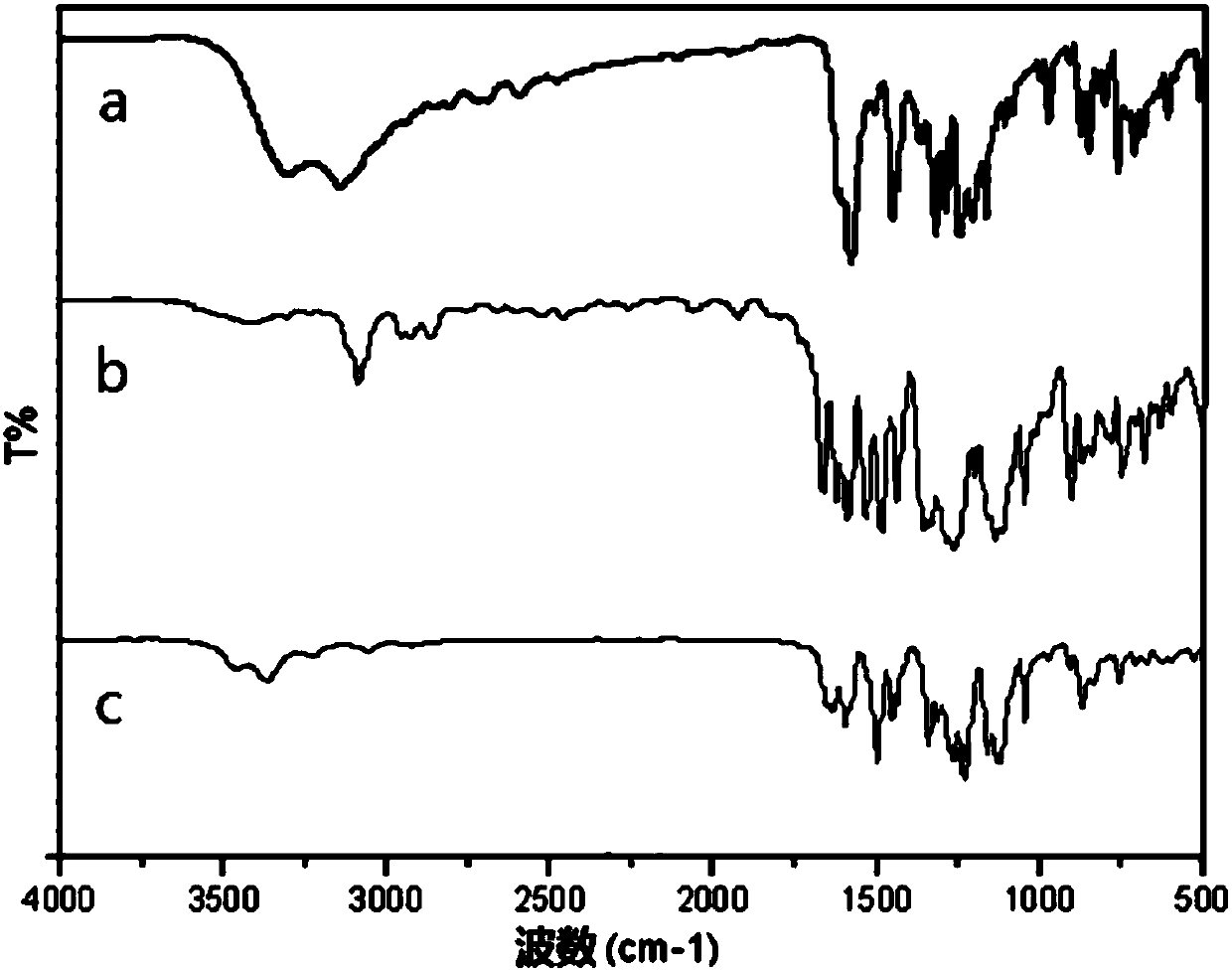
[0125] The 25mL three-necked flask is equipped with a nitrogen conduit, and the aromatic diamine represented by formula (III) and solvent N-methyl-2-pyrrolidone are added into the there-necked flask, and slowly stirred until the aromatic diamine is completely dissolved, and then the ODPA is divided into After three additions, the molar ratio of ODPA to the aromatic diamine represented by formula (III) should be strictly controlled to be 1:1, the temperature should be controlled at room temperature, and the solid content should be 15%. Afterwards, the reactant is placed in a nitrogen environment and placed at room temperature for 24 hours to obtain a viscous polyamic acid solution with a weight-average molecular weight of 20000-30000 shown in formula (I-a). Its infrared spectrum is as Image 6 Shown in a.
[0126]
[0127] Evenly coat the viscous polyamic acid reaction solution prepared above on a clean glass plate, and heat it in a muffle furnace. The heating temperature a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com