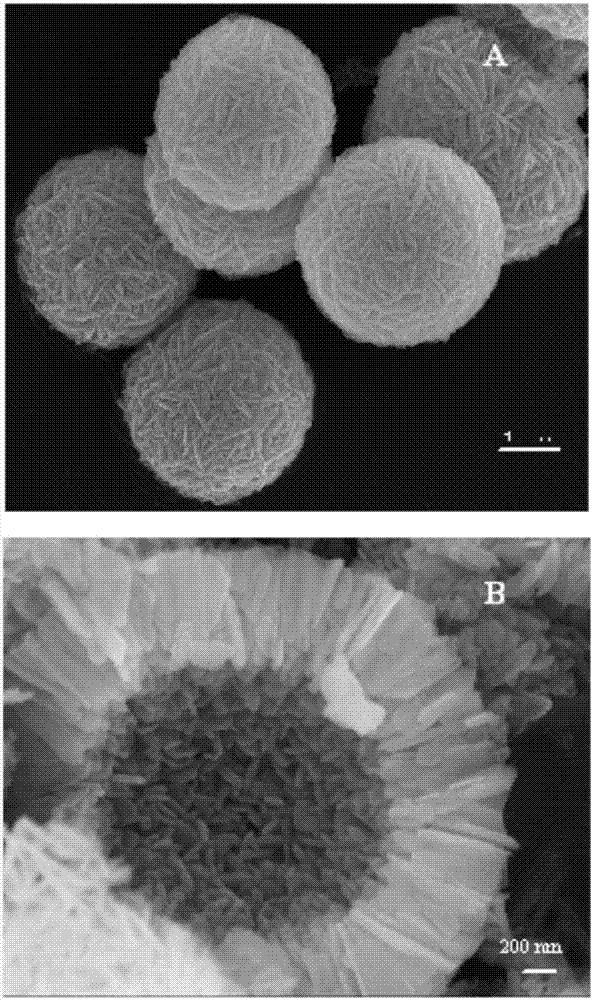
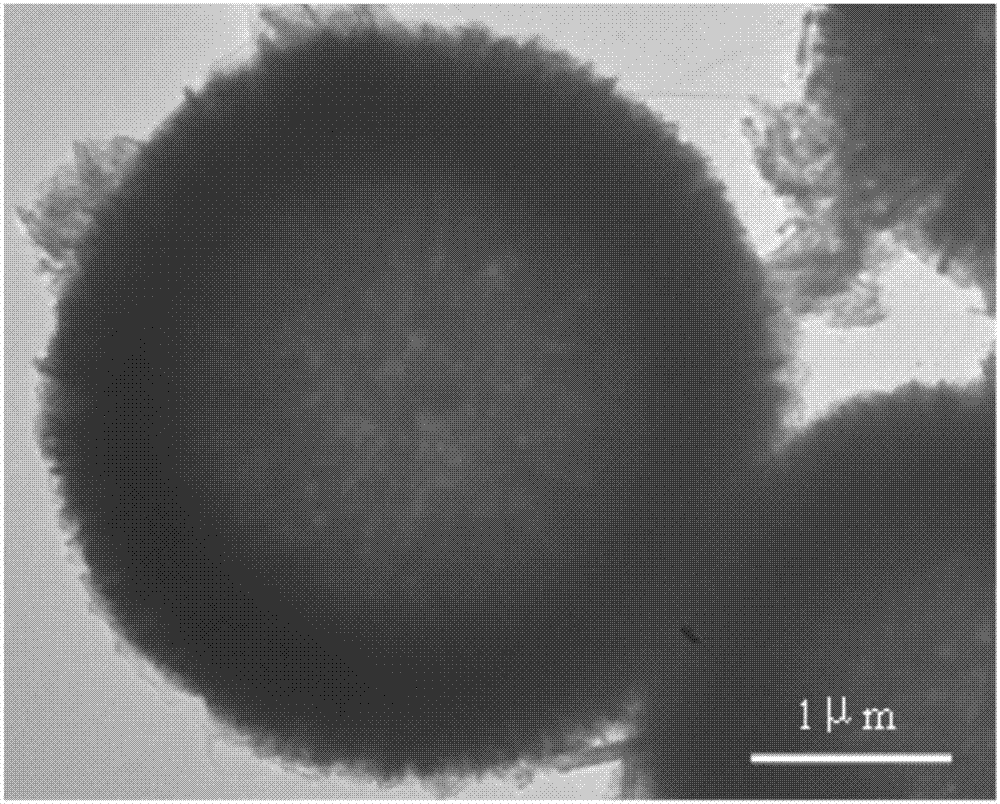
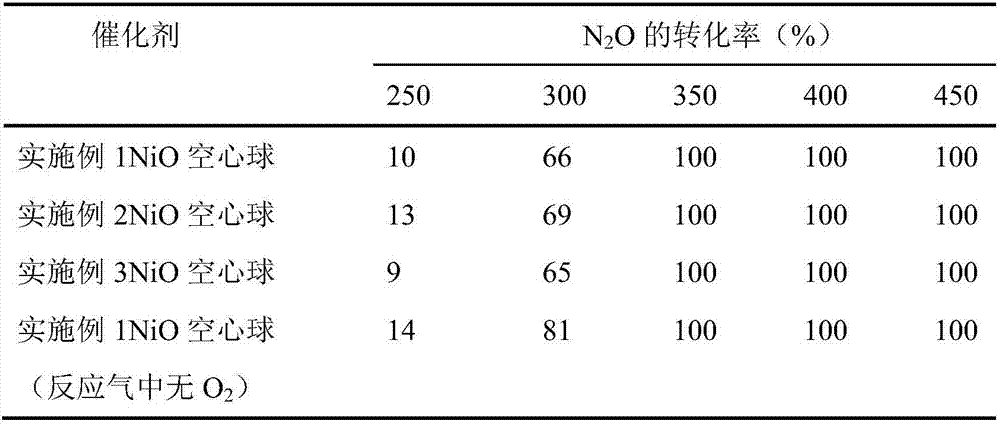
NiO hollow sphere catalyst for catalyzing N2O to directly decompose as well as preparation method and application of NiO hollow sphere catalyst
A technology of hollow spheres and catalysts, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, separation methods, etc., can solve the problems of low activity, achieve the effects of promoting adsorption, good application prospects, and avoiding complicated steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a) Take 10ml of 0.5mol / L nickel nitrate solution, 20ml of 1mol / L glycine solution and 15ml of 1mol / L sodium sulfate solution, add deionized water, add 10ml of 4mol / L hydroxide dropwise under stirring at room temperature Sodium solution, forming a blue transparent solution;
[0023] b) Stirring the blue transparent solution obtained in step a) in a water bath at 40°C for 30 minutes, transferring the resulting mixed solution to a hydrothermal reaction kettle, performing a hydrothermal reaction at 180°C for 24 hours, and then cooling down to room temperature;
[0024] c) The reaction solution obtained in step b) was suction filtered, washed, dried at 120°C for 12 hours, and then calcined in a muffle furnace at 400°C for 4 hours to prepare a NiO hollow sphere catalyst.
Embodiment 2
[0026] a) Take 10ml 1mol / L nickel nitrate solution, 10ml 2mol / L glycine solution and 15ml 2mol / L sodium sulfate solution, add deionized water, add 60ml 2mol / L sodium hydroxide drop by drop under stirring at room temperature solution, forming a blue transparent solution;
[0027] b) Stirring the blue transparent solution obtained in step a) in a water bath at 20°C for 90 minutes, transferring the resulting mixture to a hydrothermal reaction kettle, performing a hydrothermal reaction at 140°C for 36 hours, and then cooling down to room temperature;
[0028] c) The reaction solution obtained in step b) was suction filtered, washed, dried at 120°C for 24 hours, and then calcined in a muffle furnace at 350°C for 8 hours to prepare a NiO hollow sphere catalyst.
Embodiment 3
[0030] a) Take 10ml 1mol / L nickel nitrate solution, 25ml 2mol / L glycine solution and 30ml 2mol / L sodium sulfate solution, add deionized water, add 50ml 2mol / L sodium hydroxide drop by drop under stirring at room temperature solution, forming a blue transparent solution;
[0031] b) Stirring the blue transparent solution obtained in step a) in a 30°C water bath for 60 minutes, transferring the resulting mixed solution to a hydrothermal reaction kettle, performing a hydrothermal reaction at 160°C for 30 hours, and then cooling down to room temperature;
[0032] c) The reaction solution obtained in step b) was suction filtered, washed, dried at 120°C for 24 hours, and then calcined in a muffle furnace at 500°C for 4 hours to prepare a NiO hollow sphere catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com