RGD-peptide-serum protein combined segmental conjugate and preparation method thereof as well as radionuclide marker and application thereof
A radionuclide and serum protein technology, applied in the field of RGD peptide-serum protein binding fragment conjugates, can solve the problems of affecting tumor treatment effect and high side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
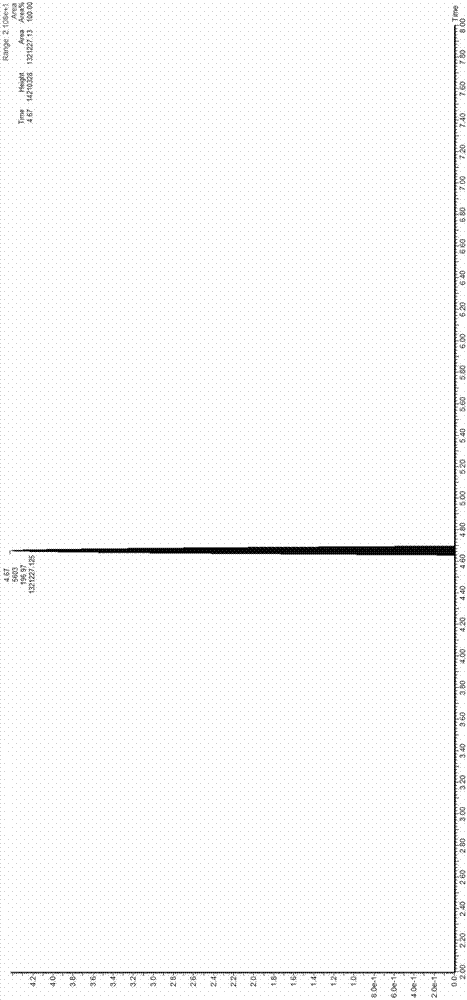
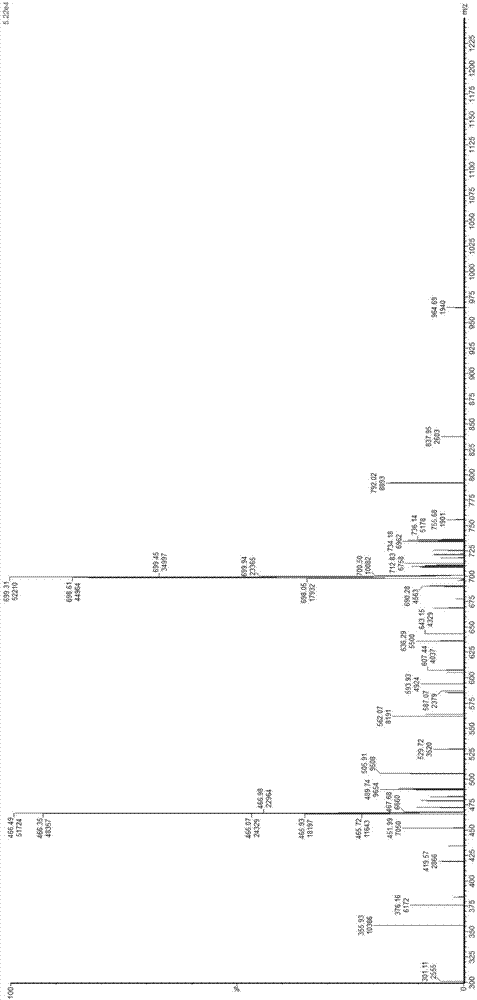
Image
Examples
Embodiment 1
[0058] PEG 4 - Preparation of c(RGDyK): using c(RGDyK) and PEG 4 To connect, the specific synthesis steps are as follows:
[0059] (1) c(RGDyK) 0.10mmol, PEG 4 0.20mmol, 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) 0.13mmol and diisopropylethylamine 0.50mmol were dissolved in 400 μL of N,N-dimethylformamide (DMF), stirred at room temperature for 1 h, and the resulting reaction solution was separated and purified by preparative high-performance liquid phase (Pre-HPLC).
[0060] (2) Add 3 mL of acetonitrile / water (volume ratio 1:1) to the above reaction solution to obtain a mixed solution, and filter the mixed solution through a 0.22 μm needle filter to obtain a clear solution.
[0061] (3) Separating and purifying the target compound using preparative high-performance liquid chromatography (Pre-HPLC).
[0062] Liquid phase parameters: preparative HPLC column (XBridge BEH C18, 21×250 mm, particle size 5 μm). Solvent A is ultrapure water...
Embodiment 2
[0104] with radionuclides 131 I, Mark III (prepared in Example 1):
[0105] (1) 100 μg / mL aqueous solution of preparation III; Na 131 1 is mixed with the solution of 100mCi / mL with physiological saline;
[0106] (2) 100μL 131 I and 100 μL III were added to a centrifuge tube coated with 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril (Iodogen), and reacted on a shaker to obtain 131 I-III radionuclide crude product solution.
[0107] (3) Purify the reaction product by HPLC, and the purification parameters are as follows:
[0108] HPLC purification parameters: HPLC column (XDB C18, 4.6×250 mm, particle size 5 μm). Solvent A is ultrapure water containing 0.1% trifluoroacetic acid, solvent B is acetonitrile containing 0.1% trifluoroacetic acid, and the mobile phase gradient is as follows:
[0109]
Flow rate mL / min
A%
B%
0min
1.0
95
5
4min
1.0
95
5
30min
1.0
42
58
35min
1.0
10
90
[0110] UV ...
Embodiment 3
[0120] 131 In vitro stability test of I-III
[0121] 131 After the synthesis of I–III, take 0.5mL (about 1.3mCi) of the purified solution, add it to 3mL of fetal bovine serum, incubate at 37°C for 2h, 8h, 24h, 48h, 72h, and 120h, then perform ITLC analysis. The pure test results are as follows:
[0122] time / h
[0123] Test result: within 72 hours, 131 I-III have good stability. Its radiochemical purity was maintained above 95% as detected by TLC. Its radiochemical purity was maintained above 90% by 8h detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com