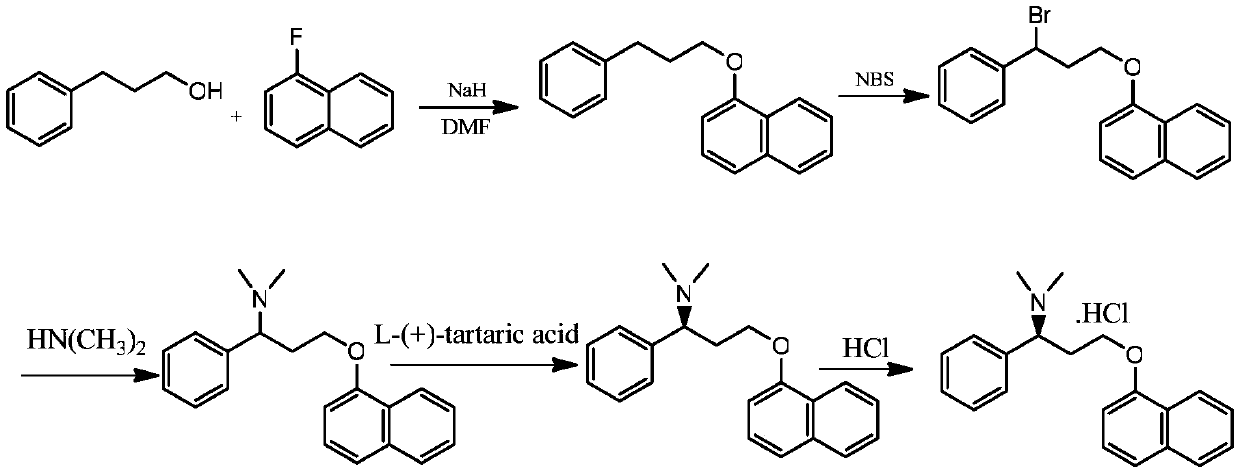
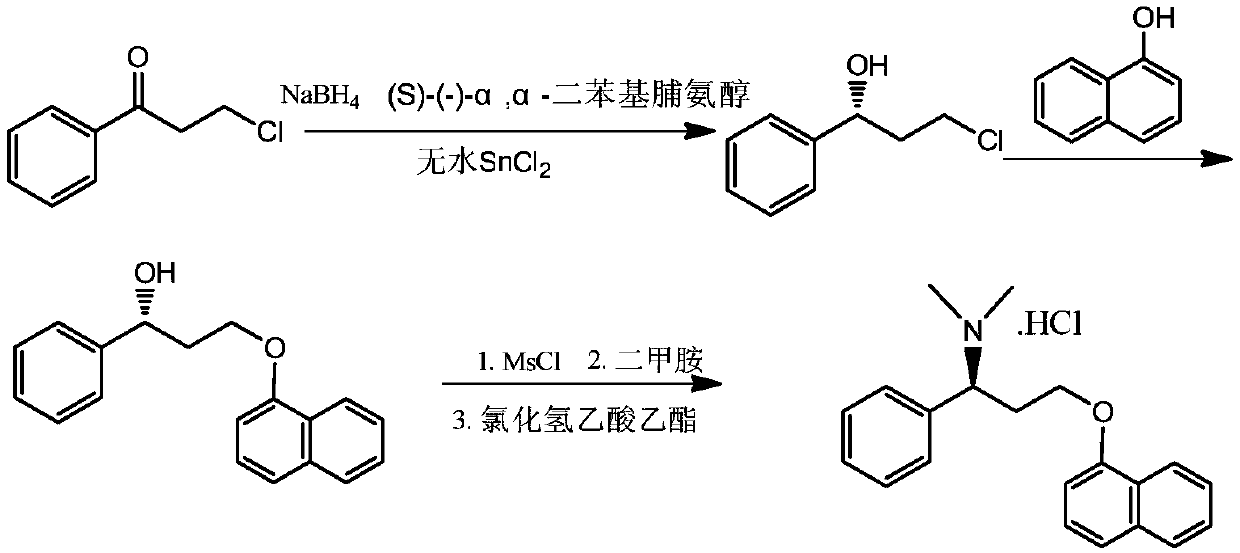
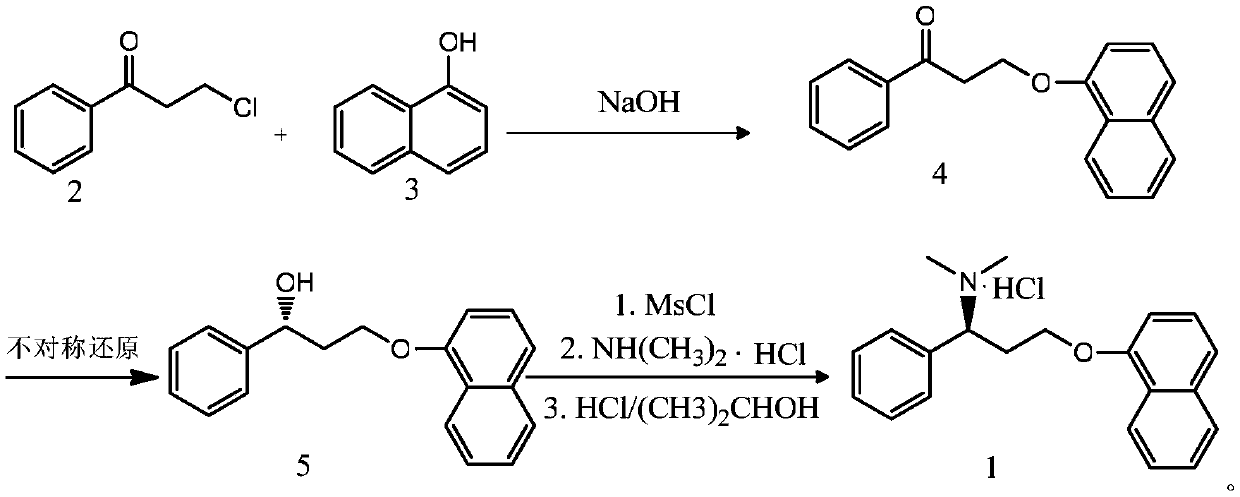
The preparation method of dapoxetine hydrochloride
A technology of dapoxetine hydrochloride and anhydrous sodium sulfate, which is applied in the field of drug preparation, can solve the problems of low optical purity of dapoxetine hydrochloride, long synthesis route steps, and immature synthesis methods, etc., and achieve easy large-scale production , easy operation and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of 3-(1-naphthyloxy)-1-phenyl-1-propanone
[0041] Weigh 8.351gK 2 CO 3 Put the solid in a 250ml three-necked flask, add 70ml N,N-dimethylformamide and blow nitrogen to maintain the nitrogen atmosphere, cool down to 0°C, add 8.002g of 1-naphthol, keep warm at 0°C for 0.5h, then add 8.508 g 3-chloropropiophenone was reacted at 15°C for 15h, then added to 250ml of water for crystallization, and suction filtered to obtain 8.997g of reddish-brown solid 3-(1-naphthyloxy)-1-phenyl-1-propanone, with a yield of 64.88 %.
[0042] 2. Preparation of (R)-(-)-3-(1-naphthyloxy)-1-phenyl-1-propanol
[0043]Dissolve 5.001g of 3-(1-naphthyloxy)-1-phenyl-1-propanone in 30ml of 1,4-dioxane, and add 21.7ml of (-)diisopinepinylborane at 80°C (1.0mol / LinTHF), after reacting at this temperature for 10h, slowly add 200ml of ice water, and stir for 30nin. The layers were left to stand, the aqueous phase was extracted with 72ml of ethyl acetate, then washed with saturated sod...
Embodiment 2
[0047] 1. Preparation of 3-(1-naphthyloxy)-1-phenyl-1-propanone
[0048] Weigh 2.417g of NaOH solid into a 250ml three-neck flask, add 70ml of N,N-dimethylformamide and blow nitrogen to maintain the nitrogen atmosphere, add 7.958g of 1-naphthol after cooling down to 0°C, and keep warm at 5°C for 5h. Then add 8.461g of 3-chloropropiophenone and react at 15°C for 40h, then add to 250ml of water for crystallization, and filter with suction to obtain 10.142g of reddish-brown solid 3-(1-naphthyloxy)-1-phenyl-1-acetone, Yield 73.14%.
[0049] 2. Preparation of (R)-(-)-3-(1-naphthyloxy)-1-phenyl-1-propanol
[0050] Dissolve 10.002g of 3-(1-naphthyloxy)-1-phenyl-1-propanone in 30ml of 1,4-dioxane, and add 31.585g of (-) diisopinepinyl boron chloride at 50°C 60% tetrahydrofuran solution of alkane, reacted at this temperature for 15h, then slowly added 200ml of ice water, and stirred for 30nin. The layers were left to stand, the aqueous phase was extracted with 72ml of ethyl acetate,...
Embodiment 3
[0054] 1. Preparation of 3-(1-naphthyloxy)-1-phenyl-1-propanone
[0055] Weigh 13.525g of KOH solid into a 500ml three-necked flask, add 250ml of N,N-dimethylformamide and blow nitrogen to maintain the nitrogen atmosphere, cool down to 0°C, add 30.728g of 1-naphthol, and keep warm at 0°C for 2h. Then add 32.671g of 3-chloropropiophenone and react at 50°C for 30h, then add to 700ml of water for crystallization, and filter with suction to obtain 39.362g of reddish-brown solid 3-(1-naphthyloxy)-1-phenyl-1-acetone, Yield 73.52%.
[0056] 2. Preparation of (R)-(-)-3-(1-naphthyloxy)-1-phenyl-1-propanol
[0057] 39.362g of 3-(1-naphthyloxy)-1-phenyl-1-propanone was dissolved in 100ml of tetrahydrofuran and nitrogen was passed through, and 60 % tetrahydrofuran A solution, react at this temperature for 20h, then slowly add 600ml of ice water, and stir for 30nin. Stand to separate the layers, extract the aqueous phase with 240ml ethyl acetate, then wash with saturated sodium bicarbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com