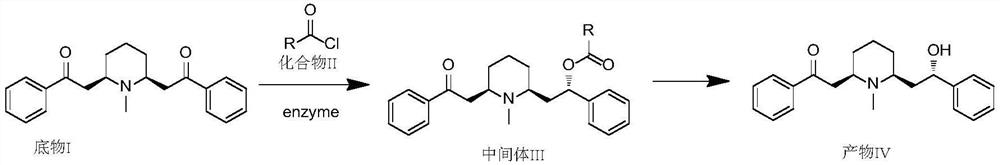
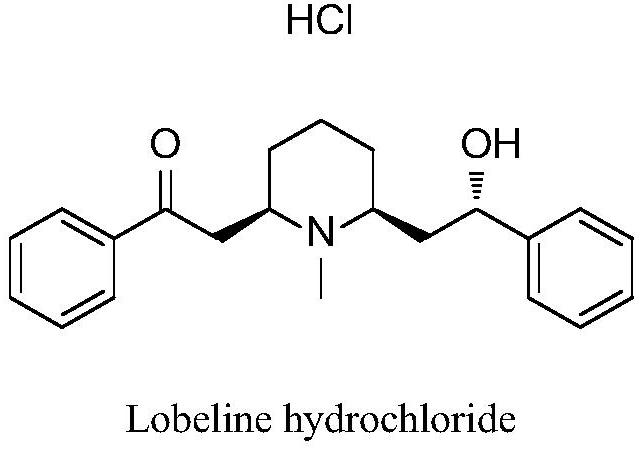
Method for synthesizing lobeline hydrochloride intermediate through enzyme catalysis chiral reduction
A technology for lobeline hydrochloride and intermediates, which is applied in the field of enzyme-catalyzed chiral reduction to synthesize lobeline hydrochloride intermediates, which can solve the problems of low selectivity of chiral reagents, long synthetic process routes, and high prices, and achieve mild conditions , The method is simple, the effect of reducing the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Above-mentioned two kinds of pyrrolidines are prepared into the preparation method of acid chloride:
[0041] Under the protection of nitrogen, add 100ml of dichloromethane, sodium bicarbonate (25.2g, 0.3mol), triphosgene (29.7g, 0.1mol) to the dry 250ml reaction flask, stir and cool down to 10°C, slowly add pyrrolidine (0.15 mol) in dichloromethane solution. During the dropping process, the temperature is controlled below 10°C. After the dropwise addition, the temperature was slowly raised to 25° C. and kept for 20 hours. Nitrogen pressure filtration, the filtrate was distilled under reduced pressure until there was no liquid, and the concentrated solution was spin-dried and recrystallized with toluene to obtain the target product.
[0042] Substrate 1 lobetanone can be purchased directly, or can be prepared voluntarily according to the following reaction formula:
[0043]
[0044] Baker's yeast (purchased by Japan Oriental Yeast Industry Co., Ltd.), after return...
Embodiment 1
[0049] Synthesis of Intermediate III
[0050]
[0051] Add 100g of toluene, 5g of n-butanol, and 20.0g of substrate I into a 500ml reaction flask. After the addition, control the reaction temperature at -10~-5°C. After the temperature stabilizes, slowly add (2S,4S)- 4-(diphenylphosphino)-2-((diphenylphosphino)methyl)-pyrrole-1-carbonyl chloride 32.3g, then add 40.0g of pretreated immobilized enzyme in the reaction flask, react Start immediately, keep warm at -10~-5°C, and react for about 48 hours. The central control will confirm the completion of the reaction (sampling HPLC or spot plate monitoring). After the reaction is completed, the enzyme is filtered off, and the filtrate is the toluene solution of the intermediate III.
[0052] Synthesis of Product IV
[0053] Add the filtrate from the previous step reaction to the reaction flask, bring the temperature to 0°C, then add 5% hydrochloric acid to the reaction flask to adjust the pH to 1-2, stir for 0.5 hours, then rais...
Embodiment 2
[0058] Other conditions are consistent with Example 1, compound II is replaced by (3S,4R)-3,4-bis(diphenylphosphine)pyrrole-1-carbonyl chloride, and the ratio of product IV to the reaction chiral isomer is 99.5: 0.5, yield 52.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com