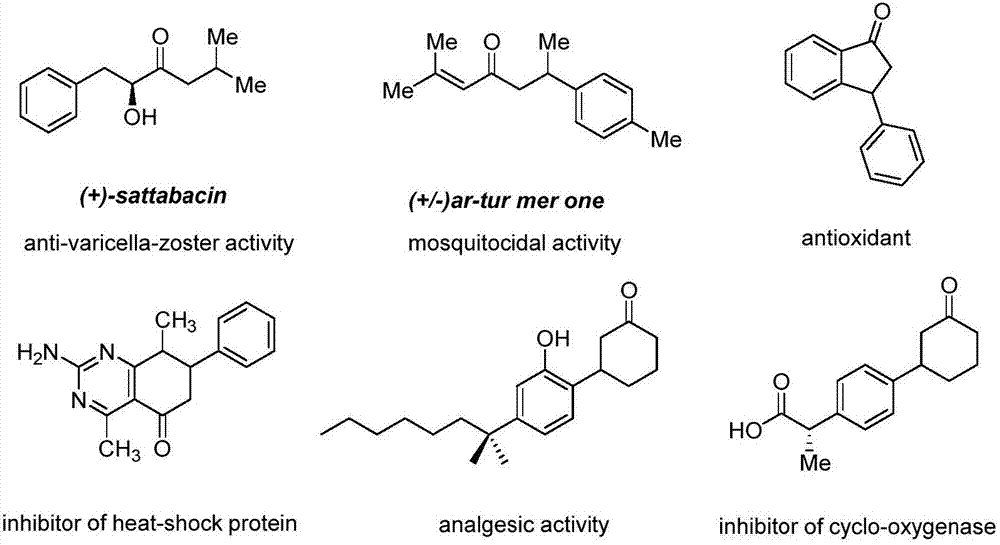
Compounding method for beta-aryl propiophenone compound
A synthesis method and compound technology are applied in the synthesis of compounds, in the field of synthesis of β-arylpropiophenone compounds, which can solve the problems of expensive precious metals, poor functional group tolerance, cumbersome experimental operations and the like, and achieve high yield and purity. Easy operation and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
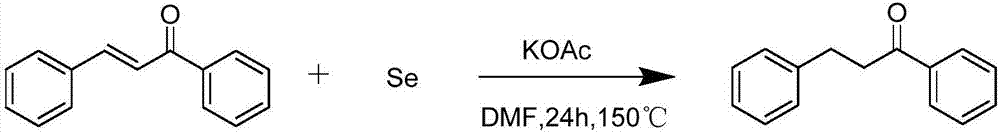
[0033] Synthesis of β-phenylpropiophenone:
[0034]
[0035] At room temperature, chalcone (0.4mmol, 1equiv), selenium powder (1.2mmol, 3equiv) and KOAc (0.8mmol, 2equiv) were added to the reaction tube, then pumped-nitrogen replaced three times, and 2mL DMF was added, Stir at a reaction temperature of 150°C, monitor the end of the reaction by thin layer chromatography (about 24h), cool the reaction mixture, then add ethyl acetate for dilution, concentrate under reduced pressure, and obtain the product through column chromatography (eluent: petroleum Ether and diethyl ether are mixed according to the volume ratio of 10:1), the product is a white solid, the weight is 81.4 mg, and the yield is 97%.
[0036] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0037] 1 H NMR (500MHz, CDCl 3 ): δ7.93(d, J=7.5Hz, 2H), 7.52(t, J=7.5Hz, 1H), 7.42(t, J=7.5Hz, 2H), 7.29-7.17(m, 5H), 3.27 (t, J=7.5Hz, 2H), 3.05(t, J=7.5Hz, 2H).
[0038] ...
Embodiment 2
[0041] Synthesis of 1-phenyl-3-(p-tolyl)-propanone:
[0042]
[0043] At room temperature, (E)-1-phenyl-3-(p-tolyl)-2-propenone (0.4mmol, 1equiv), selenium powder (1.2mmol, 3equiv) and KOAc (0.8mmol, 2equiv) were added into the reaction tube, then evacuate and replace with nitrogen three times, add 2mL DMF, stir at a reaction temperature of 150°C, monitor the reaction by thin layer chromatography (about 24h), cool the reaction mixture, and then add ethyl acetate to dilute , Distilled under reduced pressure, separated by column chromatography to obtain the product (eluent: petroleum ether and diethyl ether are mixed according to the volume ratio of 10:1), the product is a light yellow solid, the weight is 82.4mg, and the yield is 92%.
[0044] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0045] 1 H NMR (500MHz, CDCl 3): δ7.95(d, J=7.0Hz, 2H), 7.53(t, J=7.5Hz, 1H), 7.43(t, J=7.5Hz, 2H), 7.15-7.09(m, 4H), 3.27 (t, J=7.5Hz, ...
Embodiment 3
[0049] Synthesis of 3-phenyl-1-(p-tolyl)-acetone:
[0050]
[0051] At room temperature, (E)-3-phenyl-1-(p-tolyl)-2-propenone (0.4mmol, 1equiv), selenium powder (1.2mmol, 3equiv) and KOAc (0.8mmol, 2equiv) were added into the reaction tube, then evacuate and replace with nitrogen three times, add 2mL DMF, stir at a reaction temperature of 150°C, monitor the reaction by thin layer chromatography (about 24h), cool the reaction mixture, and then add ethyl acetate to dilute , Distilled under reduced pressure, separated by column chromatography to obtain the product (eluent: petroleum ether and diethyl ether are mixed according to the volume ratio of 10:1), the product is a light yellow solid, the weight is 86.2 mg, and the yield is 96%.
[0052] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0053] 1 H NMR (500MHz, CDCl 3 ): δ7.84(d, J=8.0Hz, 2H), 7.28(t, J=7.5Hz, 2H), 7.23(t, J=7.0Hz, 4H), 7.18(t, J=7.0Hz, 1H ), 3.25(t, J=7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com