Catechol group-contained natural polysaccharide composite hydrogel carrier and preparation method thereof
A composite hydrogel and catechol-based technology, which can be used in liquid transportation, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as drug burst release, low bioavailability, and rapid disintegration of carrier structures , to achieve the effects of avoiding damage, enhancing structural stability, and good biological safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
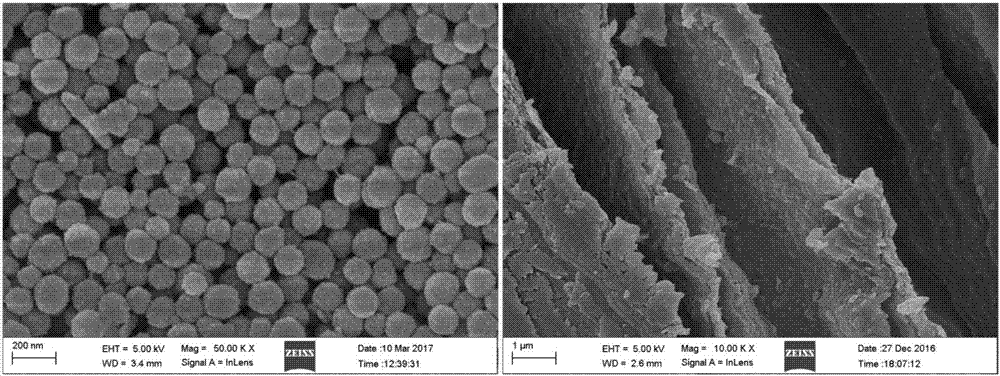
[0036] At room temperature (25° C.), 25 mg of dopamine (DA) was dissolved in 10 mL of deionized water, and blended with 50 μL of ammonia water (25%) under mechanical stirring to initiate polymerization. After 24h, the reaction was terminated, and the black precipitate was collected by high-speed centrifugation, washed and purified to obtain monodisperse polydopamine microspheres ( figure 1 (Left)). The size, distribution and surface roughness of the microspheres are regulated by the feed ratio of DA monomer and the amount of ammonia water. The polydopamine microspheres were uniformly dispersed in sodium alginate (ALG-Na) (20mg / mL) solution (wherein the mass ratio of polydopamine microspheres to sodium alginate was 1:3), and mixed with 25mg / mL calcium chloride solution Blending to prepare polydopamine / sodium alginate composite hydrogel carrier ( figure 1 (right)).
[0037] In the drug loading and in vitro release experiments, the composite hydrogel carrier can effectively ad...
Embodiment 2
[0039] At room temperature (25° C.), 25 mg of dopamine (DA) was dissolved in 10 mL of deionized water, and blended with 50 μL of ammonia water (25%) under mechanical stirring to initiate polymerization. After 24 hours, the reaction was terminated, and the black precipitate was collected by high-speed centrifugation, washed with water, and purified to obtain monodisperse polydopamine microspheres with an average particle size greater than 150 nm. The size, distribution and surface roughness of the microspheres are regulated by the feed ratio of DA monomer and the amount of ammonia water. The polydopamine microspheres were uniformly dispersed in carboxymethyl chitosan (CMCS) (40 mg / mL) solution (wherein the mass ratio of polydopamine microspheres to carboxymethyl chitosan was 1:3), and mixed with 25 mg / mL calcium chloride solution to prepare polydopamine / carboxymethyl chitosan composite hydrogel.
[0040] In drug loading and in vitro release experiments, the composite hydrogel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com