L-type lectin of litopenaeus vannamei as well as coding gene and application of L-type lectin
A technology of vanabine and lectin, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
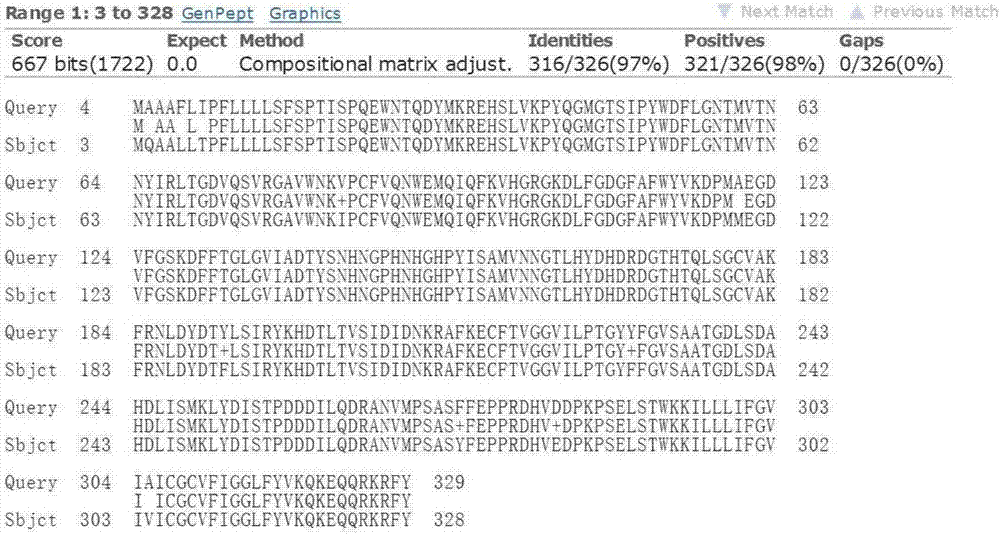
[0031] Example 1: Cloning, sequencing and sequence analysis of the L-type lectin gene lecV of Litopenaeus vannamei
[0032] (1) Primer design
[0033] Part of the L-type lectin gene sequence was obtained according to the transcriptome data of Litopenaeus vannamei in our laboratory, and primers for 5' / 3' RACE were designed according to this part of the sequence (see Table 1).
[0034] (2) Extraction of RNA
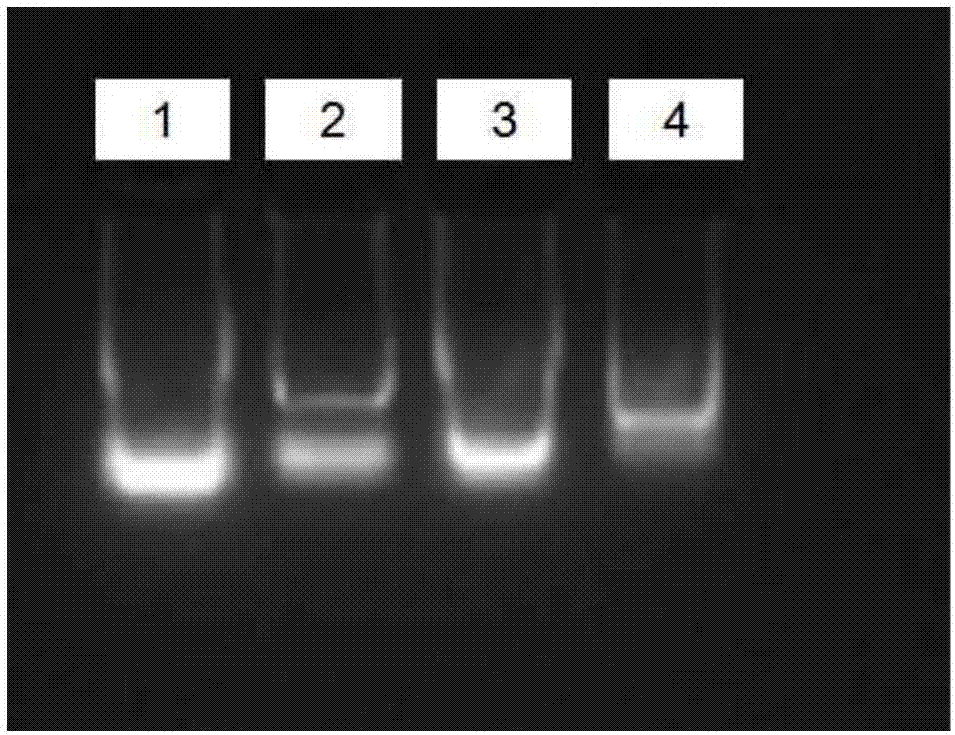
[0035]Total RNA was extracted from gill tissue of Litopenaeus vannamei by Trizol method. Operation steps: Put appropriate amount of ultra-low temperature frozen gill tissue blocks into a 1.5mL centrifuge tube, add 500μL of Trizol extract (invitrogen), and extract RNA according to the instructions of Trizol extract. RNA electrophoresis results such as figure 1 shown.
[0036] (3) Synthesis of cDNA, amplifying the cDNA of gene lecV and cloning and sequencing
[0037] 1) 3' RACE
[0038] Using the extracted gill tissue RNA of Litopenaeus vannamei as a template, PrimeScr...
Embodiment 2
[0048] Example 2: Construction of prokaryotic recombinant expression vector pET28b-lecV
[0049] The prokaryotic expression vector pET28b was amplified by primer pET28b-F and primer pET28b-R. Operation steps: The total volume of the PCR reaction system is 50 μL: prokaryotic expression vector pET28b 2 μL, primer pET28b-F 1 μL, primer pET28b-R 1 μL, Primestar DNA polymerase master mix (TaKaRa, China) 25 μL, add ddH 2 O to make up to 50 μL. The reaction conditions were: 98°C for 2.5 min; 35 cycles of denaturation at 98°C for 10 sec, annealing at 56°C for 25 sec, and extension at 72°C for 5 min; and reaction at 72°C for 10 min. The above PCR product was purified using a PCR product purification kit (TaKaRa, China) to obtain a linearized pET28b vector.
[0050] The L-type lectin gene lecV of Litopenaeus vannamei was amplified with primers LectinEx-F and LectinEx-R. Specific operation steps: The total volume of PCR reaction system is 50 μL: cDNA template 2 μL, primer LectinEx-F 1...
Embodiment 3
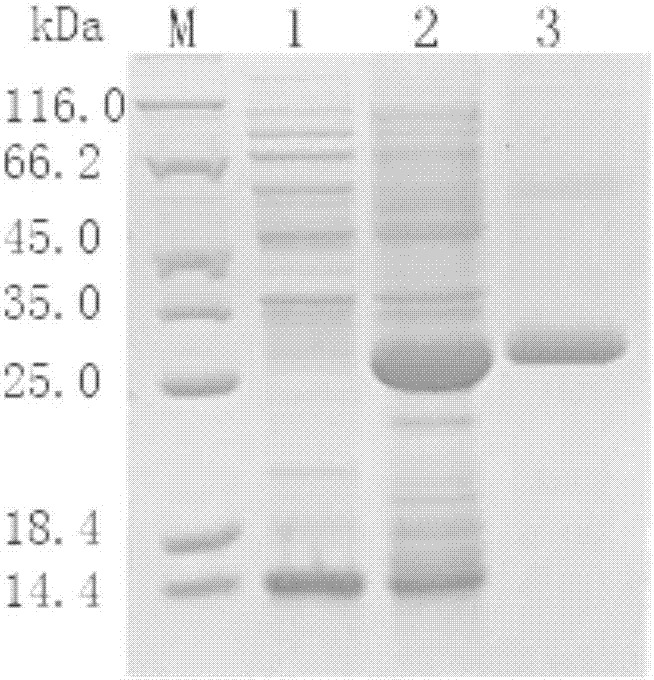
[0054] Example 3: E.coli BL21 (pET28b-lecV) induced expression and purification of LecV protein
[0055] Take 100 μL of the cultured E.coli BL21 (pET28b-lecV) in 4 mL of liquid LB medium and culture it on a shaker at 37°C until the OD 600 When it reached 0.6, IPTG was added to induce, and the bacterial solution without IPTG was used as a control. The working concentration of IPTG was 0.2mM, and the induction time was 6h.
[0056] 1 mL of samples without IPTG induction and samples induced with IPTG were taken for total protein analysis. The specific steps are: take 1 mL of bacterial liquid and centrifuge at 12,000 rpm for 3 min, discard the supernatant, and add 200 μL of ddH 2 O To resuspend the bacterial solution, take 15 μL of the bacterial solution and add 4 μL of 5×protein loading buffer, heat in boiling water for 4 minutes, and store at -20°C.
[0057] SDS-PAGE electrophoresis detection and analysis of protein samples. Specific steps: Prepare separating gel and stackin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com