Culture medium, culture medium composition, and preparation methods of culture medium and culture medium composition
A culture medium and composition technology, applied in the field of microorganisms, can solve the problems that fungal spores and bacterial spores cannot be killed, the balance of dry powder medium components is not ideal, and high-pressure sterilization cannot be used to achieve saving preparations The effects of time, medium composition uniformity, and ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The invention also discloses a preparation method of a culture medium composition, comprising the following steps:
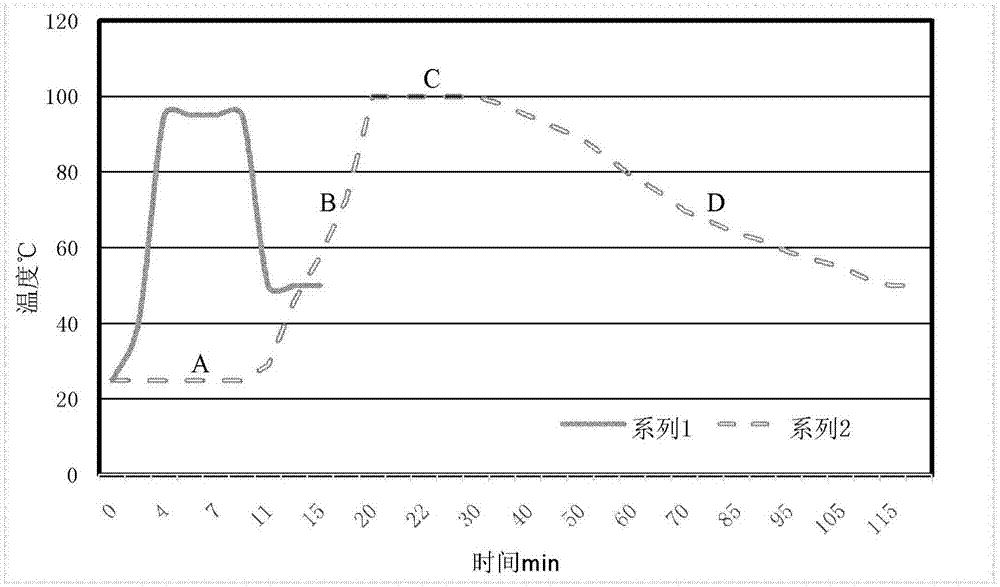
[0053] The structural support component (usually agar) in the culture medium is added with water to prepare a gel, which is packaged separately after sterilization, or packaged separately and then sterilized at high temperature to form the first component of the medium composition;
[0054] If it contains, the components that are unstable to heat, unstable under light, or unstable in the solution state in the medium are separately encapsulated into the third component of the medium composition according to the physical and chemical characteristics; The components are mixed and kept at a certain temperature (usually 45-55°C, preferably 50-55°C) before adding. When adding, the sterility of the third component can be ensured by methods such as sterile filtration. Substances that are unstable in solution need to be quantitatively dissolved to prepare a conce...
Embodiment 1
[0073] Taking SS agar as an example, the specific implementation process of the present invention is described as follows:
[0074] The SS agar recipe is as follows:
[0075]
[0076] The degree of concentration is determined according to the content of each component of the medium, and it is necessary to ensure that the content of each component after mixing is consistent with the specified component content of the preparation of the medium:
[0077] Weigh 13.5g of agar, add 400ml of pure water to prepare the first component: agar gel;
[0078] Weigh 5g of beef extract powder, 5g of peptone, 10g of lactose, 8.5g of sodium thiosulfate, 8.5g of bile salt, 8.5g of sodium citrate, 0.025g of neutral red, and 0.00033 of brilliant green, and dissolve them in 590ml of pure water to prepare the No. Two components: nutrient solution;
[0079] Weigh 1 g of ferric ammonium citrate and dissolve it in 10 ml of pure water to prepare the third component: unstable additive.
[0080] Amo...
Embodiment 2
[0085] Taking blood agar as an example, the specific implementation process of the present invention is described as follows:
[0086] The formula of blood agar basal medium is as follows:
[0087] unit g / L
[0088] 15g of peptone, 4 grams of beef extract powder, 5 grams of sodium chloride, 4 grams of yeast extract powder, 15 g of agar, 50 ml of 5% fresh defibrated sheep blood.
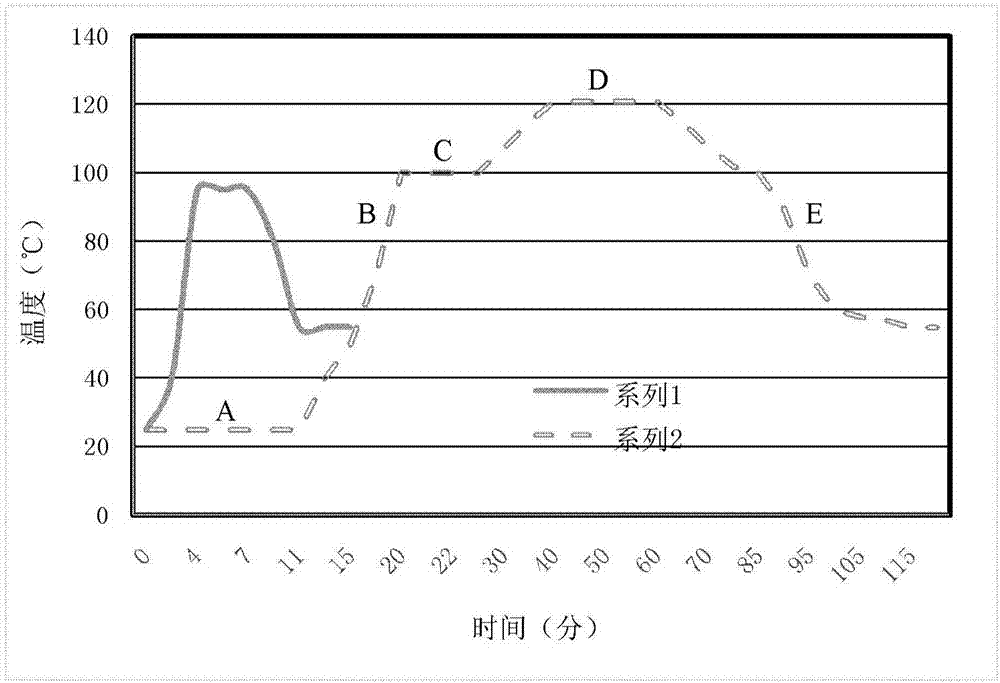
[0089] Weigh 15g of agar, add 400ml of pure water to prepare the first component: agar gel;
[0090] Weigh 15g of peptone, 4 grams of beef extract powder, 5 grams of sodium chloride, and 4 grams of yeast extract powder and dissolve them in 550ml pure water to prepare the second component: nutritional components;
[0091] Take 50 ml of 5% fresh defibrated sheep blood, and prepare it as the third component: the unstable additive component.
[0092] Both the first component and the second component are packaged separately, sterilized under high pressure at 121°C to reach a sterile state, and the third c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com