A kind of antiretroviral pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of water and heat instability, decreased dissolution, easy degradation, etc., and achieve the effects of increasing yield and drug loading, improving stability, and reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Prescription: as shown in Table 1-2
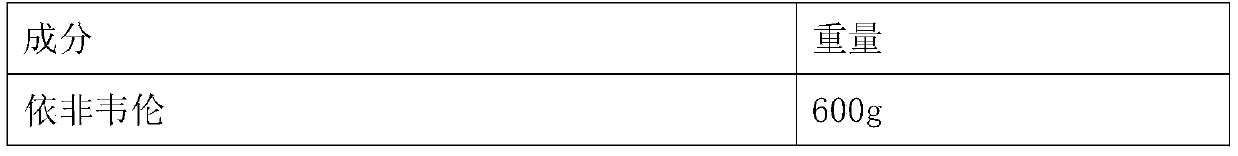
[0030] The formula of table 1 efavirenz pellet
[0031] Element weight Efavirenz 600g Sodium dodecyl sulfate 25g microcrystalline cellulose 18g Croscarmellose sodium (additional) 48g
[0032] The formula of table 2 triple preparation
[0033]
[0034]
[0035] The preparation method is as follows:
[0036] (1) Preparation of tenofovir disoproxil fumarate-β-cyclodextrin inclusion compound (saturated aqueous solution method)
[0037] Weigh 300g of β-cyclodextrin in 1.8L water, stir to dissolve it, and make a saturated solution, weigh 300g of tenofovir disoproxil fumarate to the β-cyclodextrin saturated solution at a mass ratio of 1:1 , the stirring temperature was 35°C, and the stirring was continued for 4 h at a rotational speed of 1000 r / min. After cooling and standing for 2 h, the filtrate was dried to constant weight, and the obtained white substance was the desired clathrate.
[00...
Embodiment 2
[0045] Prescription: as shown in Table 3-4
[0046] The formula of table 3 efavirenz pellets
[0047]
[0048]
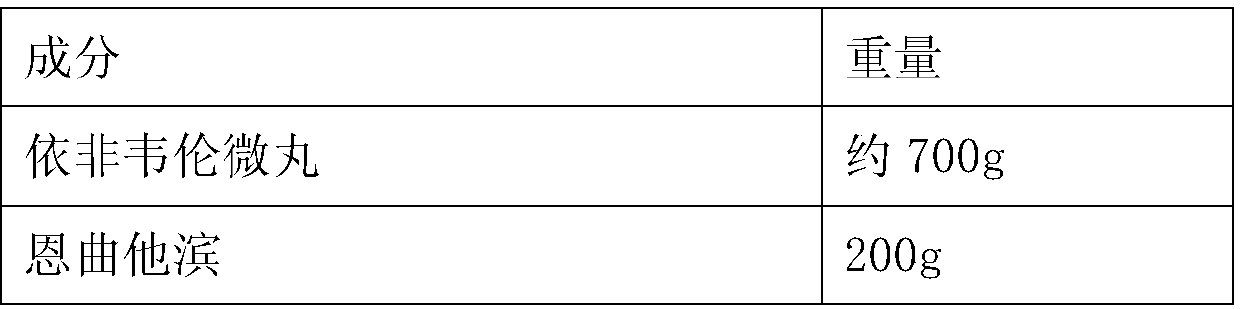
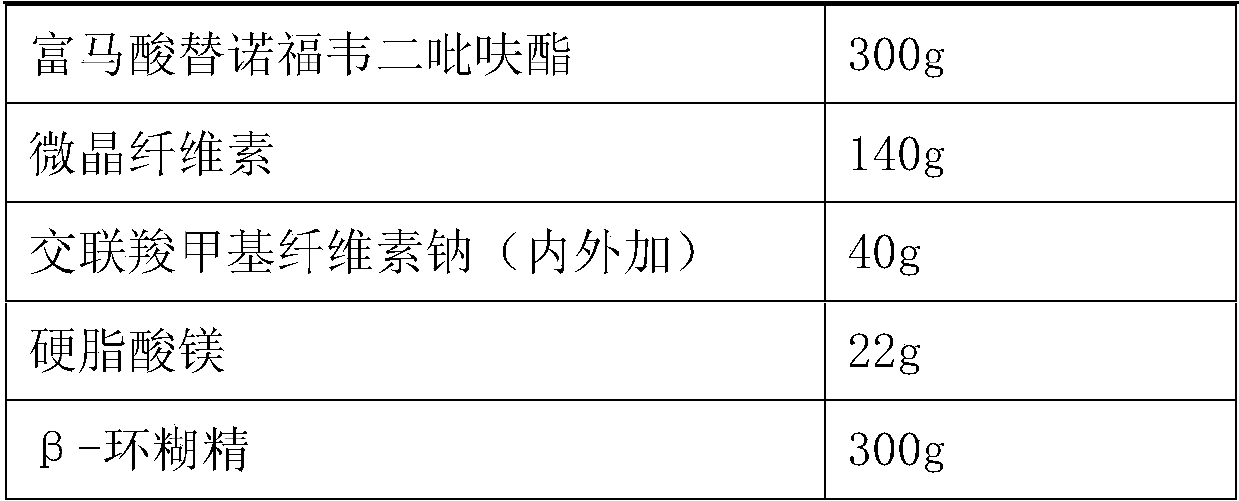
[0049] The formula of table 4 triple preparation
[0050] Element weight Efavirenz pellets about 700g Emtricitabine 200g tenofovir disoproxil fumarate 300g pregelatinized starch 130g Croscarmellose sodium (externally added) 40g Magnesium stearate 20g Micropowder silica gel 12g β-cyclodextrin 300g
[0051] (1) Preparation of tenofovir disoproxil fumarate-β-cyclodextrin inclusion compound (saturated aqueous solution method)
[0052] Weigh 300g of β-cyclodextrin in 1.8L water, stir to dissolve it, and make a saturated solution, weigh 300g of tenofovir disoproxil fumarate to the β-cyclodextrin saturated solution at a mass ratio of 1:1 , the stirring temperature was 35°C, and the stirring was continued for 4 h at a rotational speed of 1000 r / min. After cooling and standing for 2 h, the filtrate was d...
Embodiment 3
[0060] Prescription: with embodiment 1
[0061] The preparation method is as follows:
[0062] (1) Preparation of tenofovir disoproxil fumarate-β-cyclodextrin inclusion compound (grinding method)
[0063] Weigh tenofovir disoproxil fumarate 300g, weigh 300g β-cyclodextrin according to the mass ratio of 1:1 and place it in a mortar, add 10L ethanol as a wetting agent, grind in a grinder for 1h, ethanol It is ready for use after being removed by volatilization.
[0064] (2) Preparation of efavirenz pellets
[0065] Dissolve 25g sodium lauryl sulfate in 120g pure water, premix 600g efavirenz, 18g microcrystalline cellulose and prepared sodium lauryl sulfate under stirring, add 48g cross-linked carboxymethyl cellulose to the premix Sodium plain, adding water to make soft materials into balls to obtain efavirenz drug-containing pill cores, passing through a 50-60 mesh sieve for later use;
[0066] (3) Preparation of Emtricitabine Microparticles
[0067] First get 25g of crosca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com