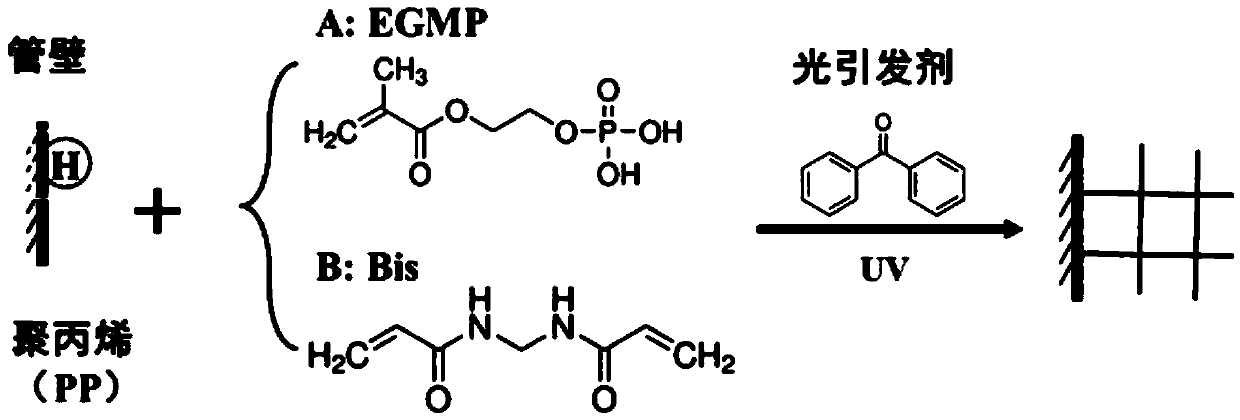
Preparation of a kind of organic monolithic small column and organic monolithic small column and application
A holistic and organic technology, applied in solid adsorbent liquid separation, chemical instruments and methods, separation methods, etc., can solve the problem that centrifugal enrichment cartridges are not used, ZipTip cartridges are expensive, and polydopamine lacks chemical stability, etc. problem, to achieve the effect of enrichment ability, short time-consuming and rapid analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation process of Ti(IV)-IMAC organic monolithic column
[0038] Such as figure 1 As indicated, a 0.10 g / mL benzophenone solution was prepared using a methanol / 1,4-butanediol solution with a volume ratio of 1:1. Take 10 μL of benzophenone solution and add it to 20 μL GE Loader tip (Eppendorf, Germany), place it in a UV generator (XL-1500A, Spectronics, New York, USA), and irradiate the reaction under 365nm wavelength ultraviolet light for 15 minutes After the reaction is over, centrifuge to remove the benzophenone solution in the GE Loader tip; repeat the above operation three times to fully activate the polypropylene column. The composition of the prepared polymerization solution is as follows: 13.4% EGMP (ethylene glycol methacrylate), 0.10g / mL Bis (methylene bisacrylamide), 45.0% DMSO (dimethyl sulfoxide), 8.3% DMF (dimethylformamide), 33.3% dodecanol; 3 μL of polymer solution was ultrasonically degassed for 10 minutes to exclude oxygen, then added to the abov...
Embodiment 2
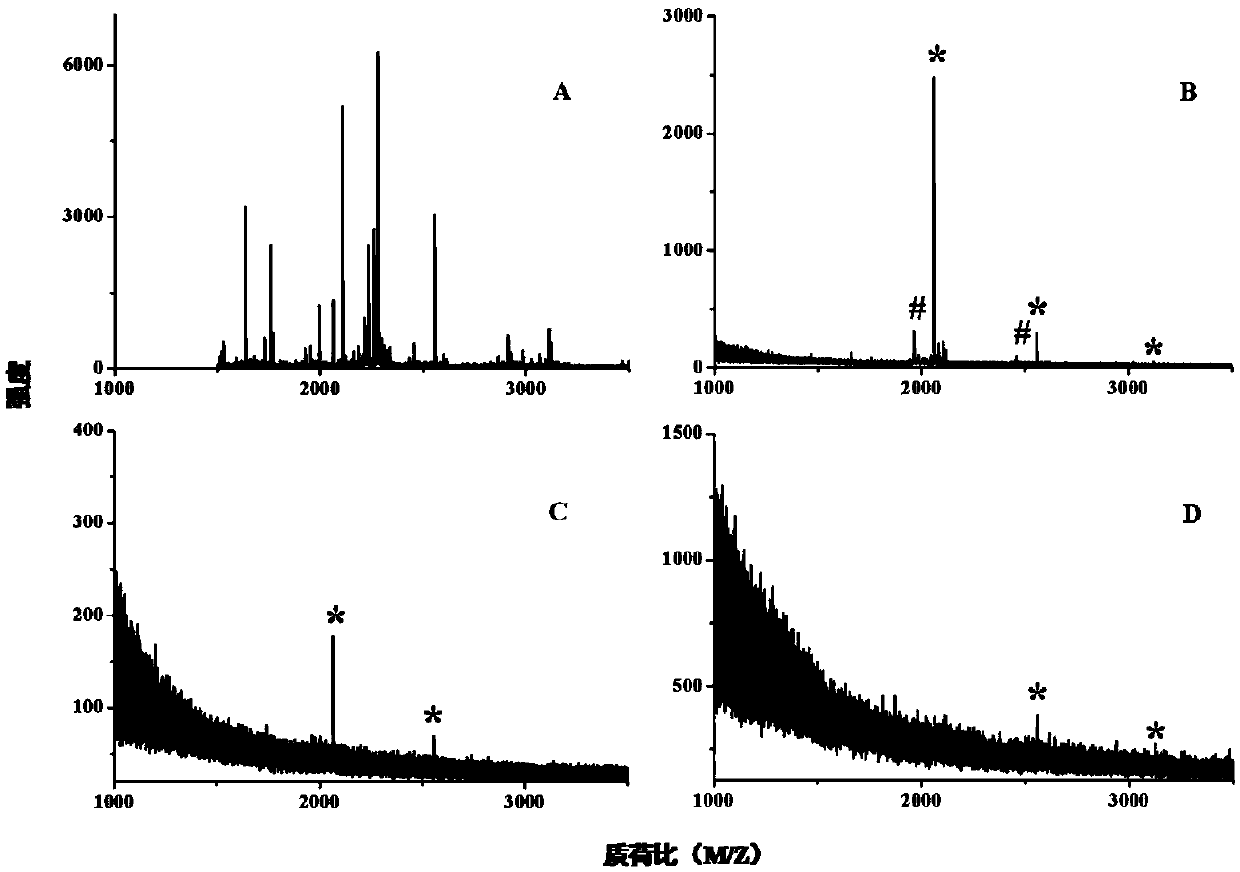
[0043] The operation process is the same as the specific process of the Ti(IV)-IMAC organic monolithic column used for enrichment analysis above, and the prepared Ti(IV)-IMAC organic monolithic column is used for the enrichment analysis of the phosphorylated protein β-casein. The trypsin hydrolyzate of 100 fmol β-casein was enriched for phosphorylated peptides. After the non-specific adsorption on the material was completely removed, the phosphorylated peptides were eluted with 10% ammonia solution, and 0.5 μL of it was eluted. The liquid was spotted on the MALDI target chip for MALDI-TOF MS analysis. As a control experiment, MALDI-TOF MS analysis was performed in parallel on the trypsin hydrolyzate of 100 fmol β-casein without the phosphopeptide enrichment step.
[0044] Such as image 3 As shown in .A, in the mass spectrum of the sample without the enrichment step, the high-intensity non-phosphopeptide peak occupies the entire spectrum, and it is impossible to identify the ...
Embodiment 3
[0046] The operation process is the same as the above Ti(IV)-IMAC organic monolithic column used for the specific process of phosphopeptide enrichment analysis. The β-casein hydrolyzate and BSA enzymolyzate are mixed in different molar ratios (500:1 and 1000:1, mol / mol) mixture to simulate complex samples to further evaluate the specificity of Ti(IV)-IMAC organic monolithic cartridges.
[0047] Such as Figure 4 As shown, under the condition of a large excess of non-phosphorylated peptides (BSA hydrolyzate), only clear mass spectrum peaks of phosphopeptides and their dephosphorylated counterparts appeared in the MALDI spectrum, even when β-casein was digested When the molar ratio of the solution and the BSA enzymolysis solution reached 1000:1, the interference signal of the non-phosphorylated peptide was still not obvious, which proved the high enrichment specificity of the Ti(IV)-IMAC organic monolithic column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com