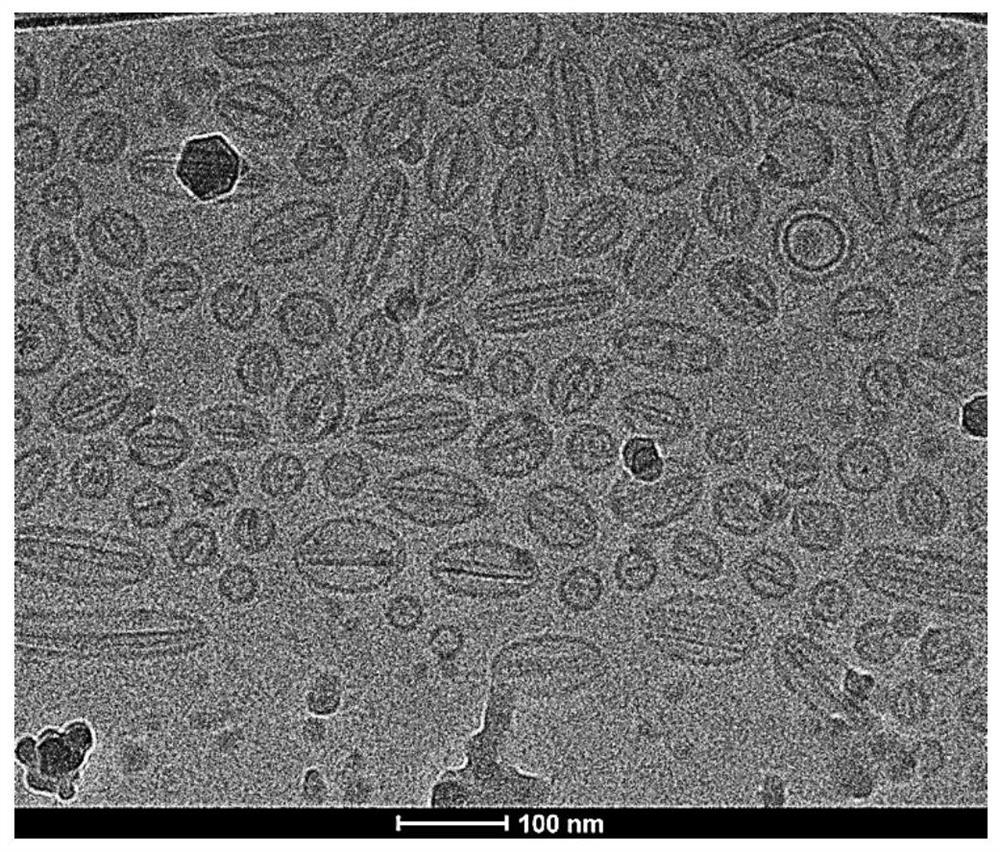
Dasatinib liposome preparation and preparation method thereof
A technology of dasatinib and nilipid, which is applied in the field of nano-drug delivery, can solve the problems of high frequency of medication, large effective dose, and limited bioavailability, and achieve improved encapsulation efficiency, good biocompatibility, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation method of Dasatinib liposome preparation
[0073] The preparation method of dasatinib liposome preparation of the present invention, comprises the steps:
[0074] (1) prepare the blank liposome that inner water phase and outer water phase all contain ammonium saline solution;
[0075] (2) preparing blank liposomes whose inner water phase contains an ammonium salt solution and whose outer water phase contains a physiological isotonic solution;
[0076] (3) Mix the blank liposome obtained in step (2) with dasatinib soluble saline solution, incubate, remove free dasatinib soluble salt, and obtain dasatinib liposome.
[0077] In step (1) and step (2), the concentration of the ammonium salt in the ammonium salt aqueous solution may be 50-500 mM. The concentration of the ammonium salt in the ammonium salt aqueous solution may also be 50-300 mM. The concentration of the ammonium salt in the ammonium salt aqueous solution may also be 300-500 mM.
[0078] The...
Embodiment 1
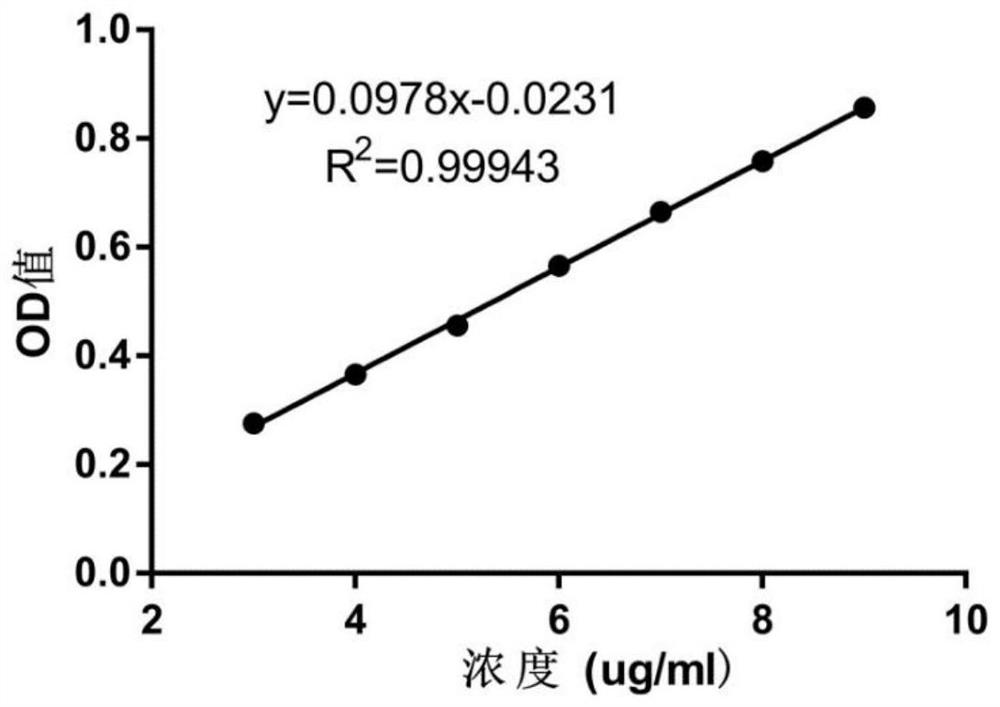
[0095] Embodiment 1, establish dasatinib detection method
[0096] In this embodiment, a standard curve of dasatinib was established, as follows.
[0097] Using ultraviolet (ultraviolet, UV) detection and analysis method: the detection instrument is TECAN 200PRO; the detection wavelength is 325nm; the detection temperature is 24°C; the detection orifice plate is 96well plates, UV-transparent; the detection volume is 200μl.
[0098] Accurately weigh 2.5002mg of dasatinib hydrochloride and dissolve it in a 25ml volumetric flask to obtain a dasatinib hydrochloride aqueous solution with a concentration of 0.1000mg / ml; mix the dasatinib hydrochloride aqueous solution with ultrapure water, Gradual dilution to obtain a series of dasatinib hydrochloride standard solutions with concentrations of 25 μg / ml, 20 μg / ml, 15 μg / ml, 10 μg / ml, 7.5 μg / ml, 5 μg / ml, and 2.5 μg / ml. The dasatinib hydrochloride standard solution of the above-mentioned concentration was detected by UV detection a...
Embodiment 2
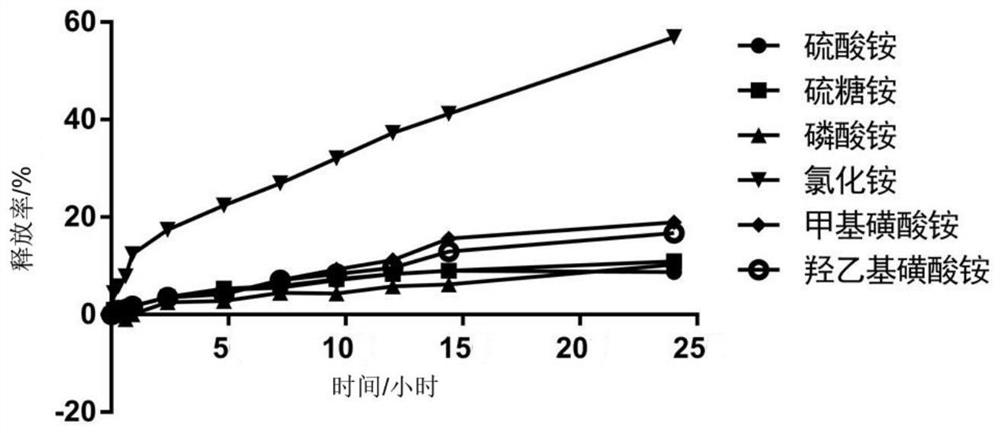
[0102] Embodiment 2, investigate the precipitation reaction of different salt solutions and dasatinib
[0103] Sodium citrate, sodium acetate, sodium sulfate, sodium chloride, ammonium sulfate, ammonium phosphate, ammonium chloride, and ammonium acetate used in this example were all purchased from Sinopharm Chemical Reagent Co., Ltd.; Ammonium isethionate and ammonium isethionate were purchased from Sigma-Aldrich.
[0104] This embodiment investigates the precipitation reaction of sodium salt solution and dasatinib, and the specific process is as follows:
[0105] (1) Precisely weigh 0.8823g sodium citrate and dissolve in 10ml double distilled water (ddH 2 (2) in, obtain the sodium citrate aqueous solution that concentration is 300mM; Accurately weigh 5.06mg dasatinib hydrochloride and be dissolved in the sodium citrate aqueous solution of 1ml 300mM;
[0106] (2) Precisely weigh 0.4082g sodium acetate and dissolve in 10ml ddH 2 In O, obtain the sodium acetate aqueous soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com