A kind of preparation method of porous cobalt phosphide nanowire catalyst
A technology of cobalt nanowires and catalysts is applied in the field of preparation of porous cobalt phosphide (CoP) nanowire catalysts, which can solve the problems of high cost, scarcity of resources, hindering large-scale production, etc. Effects of chemical activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Synthesis of linear basic cobalt carbonate;
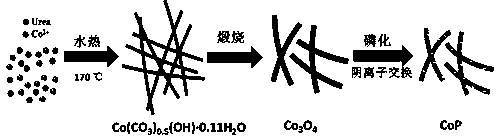
[0017] Accurately weigh 0.2g of urea and 1.124g of cobalt sulfate heptahydrate with an electronic balance, add them into a beaker containing 33ml of deionized water, stir until the solids are completely dissolved, add 7ml of glycerol, and stir at room temperature for 30min to form a solution. The resulting solution was transferred to a polytetrafluoroethylene autoclave, heated and reacted at 170°C for 24 hours to obtain a precipitate, which was filtered and washed with deionized and absolute ethanol to remove the soluble matter. Dry at 60°C, take out the product and grind to obtain linear basic cobalt carbonate.
[0018] 2. Synthesis of tricobalt tetroxide precursor;
[0019] The sample obtained above was placed in a tube furnace, heated to 400°C and calcined for 2 hours at a heating rate of 2°C / min in an air atmosphere, cooled to room temperature, and the product was taken out to obtain a tricobalt tetroxide precursor...
Embodiment 2
[0023] 1. Synthesis of linear basic cobalt carbonate;
[0024] Accurately weigh 0.2g of urea and 1.124g of cobalt sulfate heptahydrate with an electronic balance, add them into a beaker containing 33ml of deionized water, stir until the solids are completely dissolved, add 7ml of glycerol, and stir at room temperature for 30min to form a solution. The resulting solution was transferred to a polytetrafluoroethylene autoclave, heated and reacted at 170°C for 24 hours to obtain a precipitate, which was filtered and washed with deionized and absolute ethanol to remove the soluble matter. Dry at 60°C, take out the product and grind to obtain linear basic cobalt carbonate.
[0025] 2. Synthesis of tricobalt tetroxide precursor;
[0026] The sample obtained above was placed in a tube furnace, heated to 400°C and calcined for 2 hours at a heating rate of 2°C / min in an air atmosphere, cooled to room temperature, and the product was taken out to obtain a tricobalt tetroxide precursor...
Embodiment 3
[0030] 1. Synthesis of linear basic cobalt carbonate;
[0031] Accurately weigh 0.2g of urea and 1.124g of cobalt sulfate heptahydrate with an electronic balance, add them into a beaker containing 33ml of deionized water, stir until the solids are completely dissolved, add 7ml of glycerol, and stir at room temperature for 30min to form a solution. The resulting solution was transferred to a polytetrafluoroethylene autoclave, heated and reacted at 170°C for 24 hours to obtain a precipitate, which was filtered and washed with deionized and absolute ethanol to remove the soluble matter. Dry at 60°C, take out the product and grind to obtain linear basic cobalt carbonate.
[0032] 2. Synthesis of tricobalt tetroxide precursor;
[0033] The sample obtained above was placed in a tube furnace, heated to 400°C and calcined for 2 hours at a heating rate of 2°C / min in an air atmosphere, cooled to room temperature, and the product was taken out to obtain a tricobalt tetroxide precursor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com