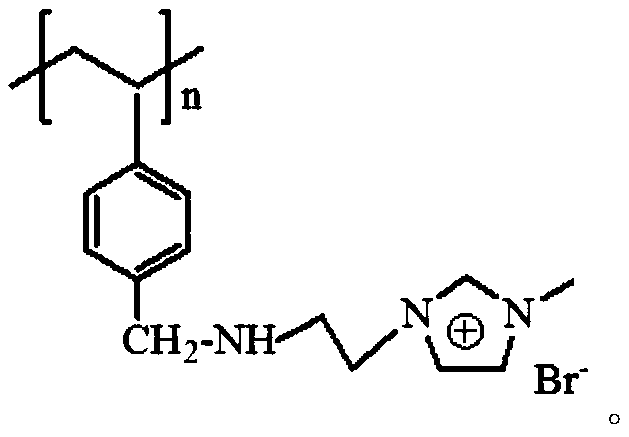
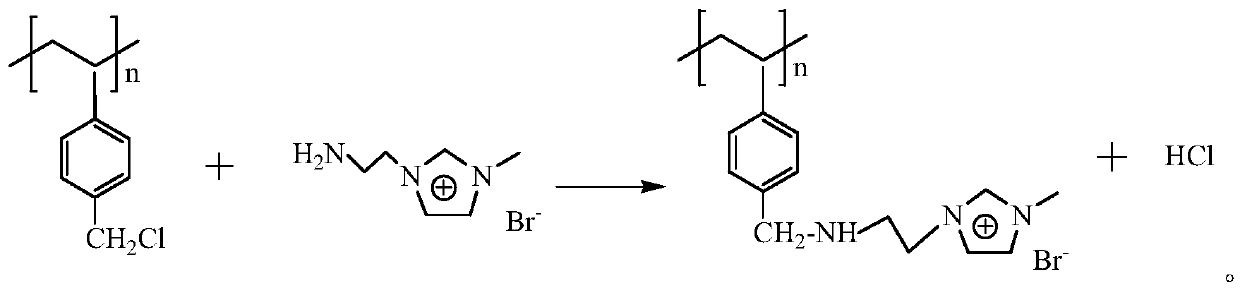
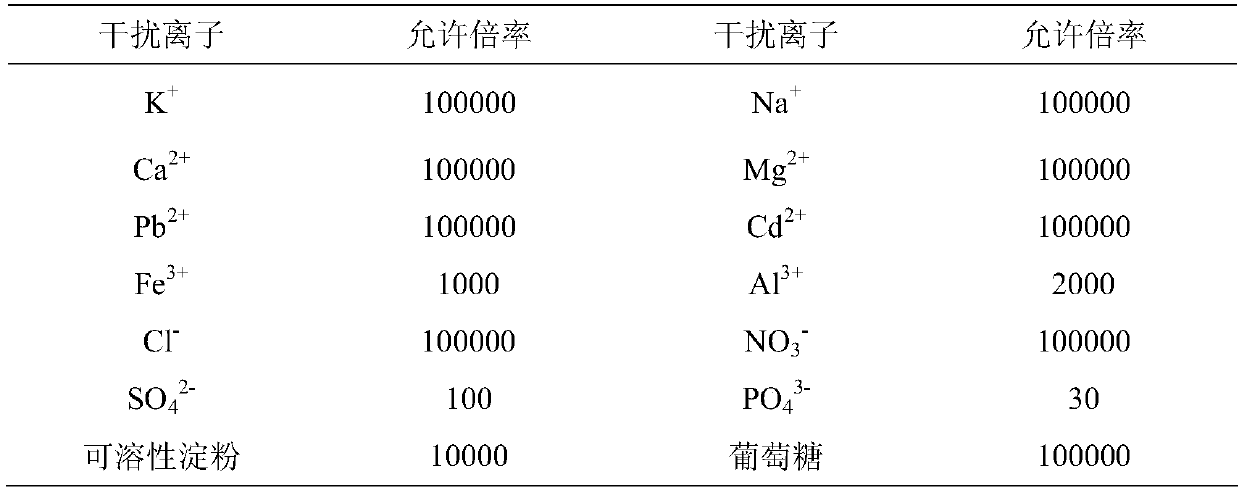
Chloromethyl polystyrene resin immobilized 1-aminoethyl-3-methylimidazolium bromide solid-phase extractant and its preparation method and application
A technology of chloromethyl polystyrene and methyl imidazolium bromide, which is applied in chemical instruments and methods, extraction water/sewage treatment, preparation of test samples, etc., can solve the mechanism of reducing selective adsorption and adsorption of pollutants and different effects, different methods and mechanisms, etc., to achieve the effect of recyclable sensitivity, good industrial application value, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 5g chloromethyl polystyrene resin and add 40mL N-methylpyrrolidone to swell for 24h, add 13.5g 1-aminoethyl-3-methylimidazolium bromide (chloromethyl polystyrene resin and 1-aminoethyl -3-Methylimidazolium bromide molar ratio 1:3) and 2.5g anhydrous K 2 CO 3, and then add 60mL N-methylpyrrolidone, seal the mixture and place it in an oil bath at 80°C for 12 hours. After the reaction, cool to room temperature, and wash the product with deionized water and absolute ethanol in turn until no 1-aminoethyl ether can be detected. Based on the absorbance of 3-methylimidazolium bromide salt and N-methylpyrrolidone, put the product in a forced air drying oven and dry it until no obvious water sample can be seen, then place it at 45°C for 24 hours in vacuum, and finally obtain The pale yellow solid chloromethyl polystyrene resin is immobilized with 1-aminoethyl-3-methylimidazolium bromide ionic liquid solid-phase extractant, and the product is sealed in a bottle and stored i...
Embodiment 2
[0037] Weigh 0.5g of chloromethyl polystyrene resin and add 10mL of N-methylpyrrolidone to swell for 12h, then add 4.6g of 1-aminoethyl-3-methylimidazolium bromide (chloromethyl polystyrene resin and 1-aminoethyl base-3-methylimidazolium bromide molar ratio 1:10) and 0.2mL triethylamine, then add 20mL N-methylpyrrolidone, seal the mixture and put it in an oil bath at 100°C for 8 hours. After the reaction Cool to room temperature, wash the product successively with deionized water and absolute ethanol until the absorbance of 1-aminoethyl-3-methylimidazolium bromide and N-methylpyrrolidone cannot be detected, and place the product in a blast drying oven After drying until no obvious water sample can be seen, place it at 45°C for 24 hours in a vacuum to obtain a light yellow solid chloromethyl polystyrene resin immobilized 1-aminoethyl-3-methylimidazolium bromide salt ionic liquid Solid phase extraction agent, the product is sealed in a bottle and stored in a desiccator for later...
Embodiment 3
[0041] Weigh 1g of chloromethyl polystyrene resin and add 10mL N-methylpyrrolidone to swell for 24h, then add 5.6g of 1-aminoethyl-3-methylimidazolium bromide (chloromethyl polystyrene resin and 1-aminoethyl -3-Methylimidazolium bromide molar ratio 1:6) and 0.5g anhydrous Na 2 CO 3 , then add 20mL of N-methylpyrrolidone, seal the mixture and place it in a 70°C oil bath for 14 hours of reaction. After the reaction, cool to room temperature, and wash the product with deionized water and absolute ethanol in turn until no 1-aminoethyl ether can be detected. Based on the absorbance of 3-methylimidazolium bromide salt and nitrogen-methylpyrrolidone, the product was dried in a forced air drying oven until no obvious water samples were seen, and then placed in vacuum at 45°C for 24 hours, and finally obtained light The yellow solid chloromethyl polystyrene resin is immobilized with 1-aminoethyl-3-methylimidazolium bromide salt ionic liquid solid-phase extractant, and the product is s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com