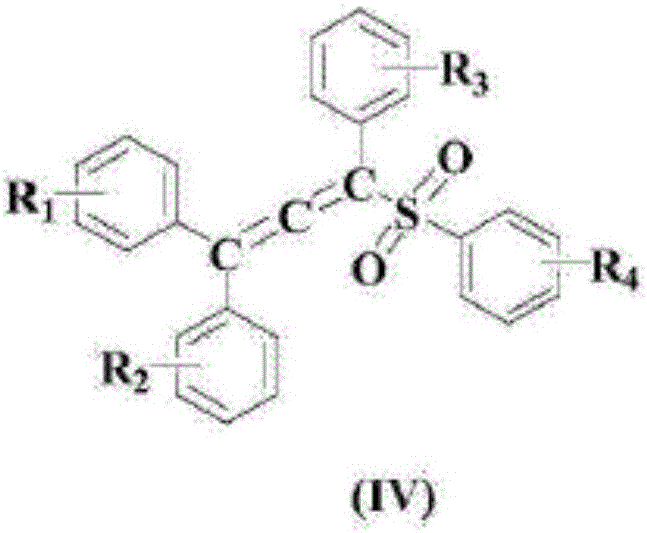
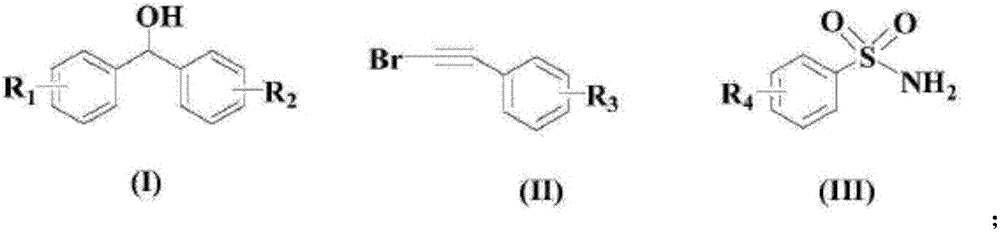
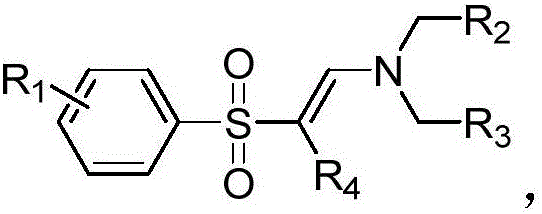
Method for synthesizing vinyl sulfone compound
A synthesis method and compound technology are applied in the preparation of organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problems of poor economy, high price, and difficulty in handling, etc. Achieving the effect of broad reaction substrate, reduced reaction cost and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the dry reaction tube, add 0.2mmol of p-toluenesulfonyl chloride, 1.0mmol of triethylamine, 3%mmol of Eosin Y, 0.6mmol K 2 HPO 4 , And then add 6mL of C 2 H 5 OH / CH 3 COCH 3 (1:2), under the 3W blue LED light, react for 2h in the air at room temperature. After the reaction, wash with water, extract, dry, and then remove the organic solvent under reduced pressure. The product undergoes thin layer chromatography and column chromatography ( Petroleum ether / ethyl acetate = 5 / 1). The resulting product yield is 67%.
[0030] (E)-N,N-diethyl-2-tosylethenamine
[0031] Brown oil.
[0032] 1 H NMR(400MHz, CDCl 3 ): δ7.75(d,J=8.2Hz,2H), 7.31(d,J=12.7Hz,1H), 7.27(d,J=8.0Hz,2H), 4.91(d,J=12.7Hz,1H ), 3.18 (broad doublet, 4H), 2.41 (s, 3H), 1.17 (bs, 6H).
[0033] 13 C NMR(100MHz, CDCl 3 )δ148.5, 142.3, 141.8, 128.5, 125.9, 91.4, 49.8(bs), 42.4(bs), 21.2, 14.5(bs)11.0(bs).
[0034] HRMS(ESI):calcd for C 13 H 19 O 2 NS+Na=276.1020,found:276.1029.
Embodiment 2
[0036] In the dry reaction tube, add 0.2mmol of benzenesulfonyl chloride, 1.0mmol of triethylamine, 3%mmol of Eosin Y, 0.6mmol K 2 HPO 4 , And then add 6mL of C 2 H 5 OH / CH 3 COCH 3 (1:2), irradiated by 3W blue LED lamp, react for 2h in the air at room temperature. After the reaction, wash with water, extract, dry and then remove the organic solvent under reduced pressure. The product is subjected to thin layer chromatography and column chromatography ( Petroleum ether / ethyl acetate = 5 / 1). The resulting product yield is 65%.
[0037] (E)-N,N-Diethyl-2-(phenylsulfonyl)ethenamine
[0038] White solid.
[0039] mp 45-46℃; 1 H NMR(400MHz, CDCl 3 )δ7.92-7.86(m,2H),7.5-7.47(m,3H), 7.35(d,J=12.7Hz,1H), 4.94(d,J=12.7Hz,1H), 3.22(broad doublet, 4H), 1.19 (broaddoublet, 6H).
[0040] 13 C NMR(100MHz, CDCl 3 )δ148.9, 145.2, 131.3, 128.6, 125.8, 91.0, 49.8(bs), 42.5(bs), 14.5(bs), 10.9(bs).
[0041] HRMS(ESI):calcd for C 12 H 17 O 2 NS+Na=257.1324, found: 257.1318.
Embodiment 3
[0043] In the dry reaction tube, add 0.2mmol of p-methoxybenzenesulfonyl chloride, 1.0mmol of triethylamine, 3%mmol of Eosin Y, 0.6mmol K 2 HPO 4 , And then add 6mL of C 2 H 5 OH / CH 3 COCH 3 (1:2), under the 3W blue LED light, react for 2h in the air at room temperature. After the reaction, wash with water, extract, dry, and then remove the organic solvent under reduced pressure. The product undergoes thin layer chromatography and column chromatography ( Petroleum ether / ethyl acetate = 5 / 1). The resulting product yield is 63%.
[0044] (E)-N,N-diethyl-2-(4-methoxyphenylsulfonyl)ethenamine
[0045] Brown oil.
[0046] 1 H NMR(400MHz, CDCl 3 )δ7.81(d,J=8.9Hz,2H), 7.31(d,J=2.5Hz,1H), 6.97(d,J=8.9Hz,2H), 4.92(d,J=12.7Hz,1H) ,3.88(s,3H), 3.20(broad doublet, 4H), 1.18(bs,6H).
[0047] 13 C NMR(100MHz, CDCl 3 )δ161.8, 148.2, 137.1, 127.9, 113.7, 91.8, 5.3, 49.7(bs), 42.1(bs), 14.4(bs), 11.0(bs).
[0048] HRMS(ESI):calcd for C 13 H 19 O 3 NS+Na=292.1005,found:292.0978.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com