Allyl-containing polyimide diamine monomer and polyimide polymer and preparation method thereof
An allyl polyimide diamine and polyimide technology, which is applied in the field of polyimide material synthesis, can solve the problems of poor material processing performance and application range, and achieve good thermal stability, mechanical properties, processing The effect of diversifying the methods and broadening the scope of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (3,3'-diallyl-4,4'-bis(4-nitro-2-trifluoromethylphenoxy)biphenyl (DNPB) is synthesized as follows:
[0041] Take by weighing 0.01mol allyl biphenyl diphenol (DABP), 0.0215mol 2-chloro-5-nitro-trifluorotoluene, 1.451g potassium carbonate (K 2 CO 3), was added to a 100mL three-necked flask with mechanical stirring, and at the same time, 30mL DMF solvent and 10mL toluene were added as a water-carrying agent. Under the protection of argon, when the temperature reached 135°C, the reflux was kept stable, and water was continuously carried for 3 hours. Toluene was released, the temperature of the reaction mixture solution was raised to 145°C, and the reaction was carried out for 10 h. Pour the reaction solution into about 500mL of ice-deionized water to obtain a khaki-yellow precipitate, wash it several times with distilled water under normal temperature mechanical stirring, filter it with suction, and dry it in a vacuum oven to obtain 4.8g of allyl-containing dinitro monome...
Embodiment 2
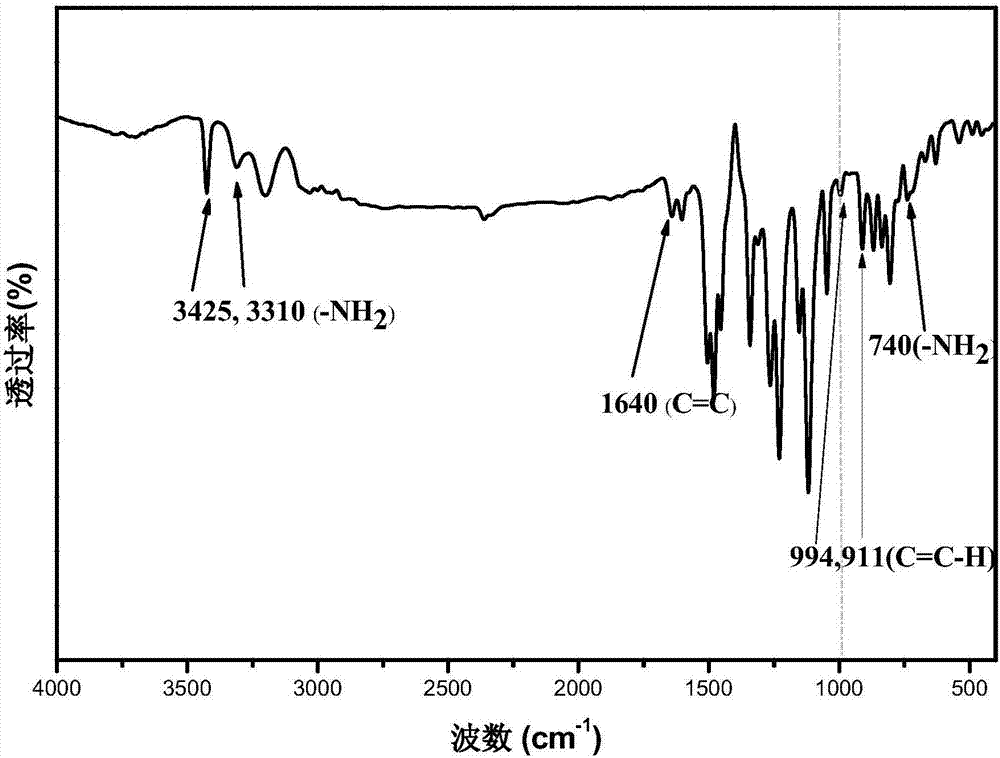
[0043] Add 4.8g (7.4mmol) DNPB into a 250mL three-neck flask, add 7g (125mmol) reduced iron powder, then add 90mL mixed solvent (30mL each of distilled water, ethanol and acetone), and mechanically stir under the protection of argon Heat until boiling begins to reflux. Slowly add 2mL of dilute hydrochloric acid dropwise into the boiling three-necked flask, keeping 5s / drop. After the dropwise addition of HCl, the reactant continued to be heated under reflux for 5 h and then the heating was stopped. Add 0.96g (24mmol) sodium hydroxide, stir for three minutes, filter the product while it is hot, recrystallize it with ethanol water, and keep 60°C for vacuum drying to obtain a yellow reduction product containing allyldiamine monomer DBDA . The measured infrared spectrum is as figure 1 shown.
Embodiment 3
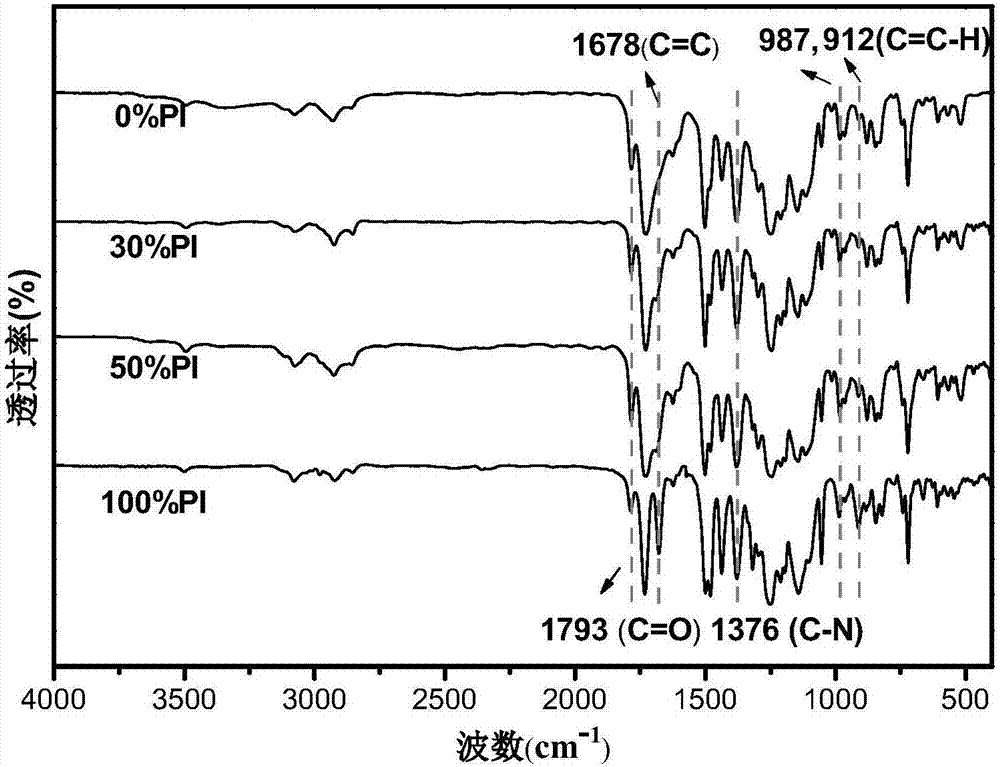
[0045] In a nitrogen environment, add the dried 0.888g (2mmol) 6FDA into a 100mL three-necked bottle connected with a drying tube, and dissolve it completely with 5mL dimethylacetamide (DMAc), then 0.4006g (2mmol) ODA and A mixture of 13.3 mL of DMAc. After the dropwise addition, the mixed solution was mechanically stirred at room temperature for 24 hours to form a viscous polyamic acid. Add 2 mL of pyridine and 4 mL of acetic anhydride to the mixture, continue to react for 8 hours at 60° C. in an oil bath, and discharge the material in absolute ethanol. At this time, white flocs appear, which are filtered and used Distilled water and ethanol were washed three times respectively, and vacuum-dried in an oven at 100° C. to obtain polyimide (0% PI) without allyl groups.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com